A Greener Future of Discovery with More Powerful Drug Screens
By Wenshu Xu & Katie Hansel

To reach its net zero targets, the pharmaceutical industry needs to overhaul processes across the entire product life cycle, including drug discovery. Katie Hansel, drug screening specialist at AstraZeneca, and Wenshu Xu, life science technology consultant at TTP, consider how “down-scaling” high-throughput screening could make a big difference.
The initial phases of drug discovery are, by necessity, “wasteful” – in the sense that, to identify the select few compounds with enough promise to go forward into biological studies, thousands or millions of other compounds undergo high-throughput screening (HTS) [1, 2]. For example, a typical screening campaign at AstraZeneca tests 2 million compounds, requiring up to 2000 1536-well plates and 12L of reagents and generating around 50kg of plastic waste in pipette tips, microplates and reagent vessels. Reducing the scale of the unit operations in drug discovery workflows will not only reduce the resource consumption associated with HTS but also unlock more powerful drug discovery strategies for the pharmaceutical industry.
Compound storage
Upstream of HTS, compound libraries require storage, maintenance, and eventual transfer for screening. This has already created opportunities to increase efficiency and reduce resource consumption. Indeed, AstraZeneca has developed a fully acoustic miniaturised compound management system for non-contact compound dispensing by storing compounds in acoustically compatible tubes in ultra-high-density trays [3, 4]. Acoustic dispensing uses a pulse of ultrasound to move nanolitre volumes of fluids without any physical contact, allowing the direct transfer of cherry-picked compounds from tubes into assay-ready plates for HTS concentration response experiments. This system increases speed of access to the compound collection, minimises footprint and process handling, and saves on sample consumption, system solvent and labware.
From microplates to microarrays
After compound transfer, standard HTS workflows are carried out in 1536-well plates using robotic pipetting systems to increase throughput and reduce waste [1, 5]. Further improvements could be made possible by down-scaling dispensing technologies to pico- or nanolitre volumes to create printed microarrays.
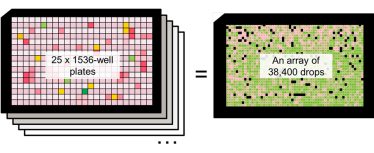
Replacing microplates with microarrays will allow more compounds to be tested in an equivalent footprint. For example, an array of 38,400 nanolitre droplets with 0.45 mm pitch can fit into the footprint of a single microplate (Figure 1). This would have a significant impact on storage, lab space, and energy consumption. Throughput can also be improved by increasing the density of the printed array as well as the speed of printing.
Going from microliter-scale in well-plates to nanoliter-scale in droplets also leads to a reduction in volume by at least a factor of 1000. This reduces reagent use and is especially beneficial when screening drugs against limited and valuable patient samples.
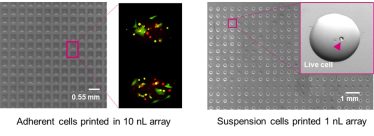
TTP’s SureDrop bio-printing is one technology that can be used to create such microarrays, precisely depositing – or “printing” – cells and biological reagents over arrays of compounds for drug discovery (Figure 2) [6]. Bio-printing workflows enhance the precision of creating these arrays, allowing for more intricate and controlled experiments. By controlling the number of cells per drop, single-cell level information, such as cell phenotypes and cell morphology, can be obtained with high-content imaging tools.
From a technology perspective, working at nanolitre volumes also raises new challenges. Minimising evaporation during and after printing is crucial, given that tiny droplets are considerably more susceptible to environmental changes than the microlitre volumes utilised in microplates. Even slight changes in droplet volume can lead to variations in compound concentrations and embedded cell viability, potentially impacting the reliability of results. When handling droplets on such a minute scale meticulous attention needs to be paid to reagent formulation, temperature, and humidity.
Bio-printed microarrays also have the potential to be seamlessly integrated into established drug discovery workflows as they can adhere to the standard SBS format. This could allow them to fit into existing microplate holders in downstream analytical instruments such as microscopes, fluorescent/luminescent plate-readers, and mass spectrometers.
Moving away from plate-based to microfluidic approaches
Still more valuable approaches to drug discovery lie beyond the reach of any plate-based method. Another way of generating droplets with high-throughput is via microfluidics, where aqueous droplets are generated in an immiscible carrier fluid [7]. The aqueous droplets can serve as picolitre-volume reaction vessels, allowing many more possibilities to be screened compared to traditional approaches.
Droplet-based microfluidic workflows have been implemented for antibody drug screening [8], directed evolution screening of enzymes [9], and DNA-encoded library (DEL) screening [10]. There are, however, technological challenges for microfluidic droplet-based screening. These include droplet cross-talk, compatibility of surfactants used to control droplet formation with biologics, limited commercially available microfluidic systems, and the development of suitable reporting analytics such as mass spectroscopy or fluorescence [11].
A particular challenge for small molecule HTS is getting a unique, tracked compound per droplet.
With appropriate technological advances, down-scaling the unit operations in HTS will not only reduce resource consumption but also open up novel drug discovery strategies. Collaboration will be crucial to harness the full potential of technologies that could lead to the discovery of drugs that will otherwise remain “hidden gems”.
Want to find out more?.
References
- Hansel et al., Drug Discovery Today, 2022, 27, 8, 2051-2056
- Wigglesworth, et al. High throughput screening: methods, techniques and applications
- Green et al., Drug Discovery Today, 2021, 26, 1, 5-9
- https://www.astrazeneca.com/r-d/our-technologies/acoustic-tube-sample-management.html
- Knight et al., SLAS Discovery, 2017, 22, 6, 732–742
- https://www.ttp.com/case-studies/worlds-first-multimodality-multiplexing-microarray-system-enabled-by-ttp-suredrop-technology/
- Moragues et al., Nat Rev Methods Primers, 2024, 3, 32
- https://www.ttp.com/case-studies/cyto-mine-single-cell-analysis-system/
- Stucki et al., Chem. Int. Ed. 2021, 60, 24368-24387
- Cochrane et al., ACS Comb. Sci. 2019, 21, 5, 425-435
- Payne et al., Lab Chip, 2020, 20, 2247-2262