Spotlight on upconversion nanoparticles: finding the right chemistry to make life science applications “click”
By Wenshu Xu & Verity Jackson
Biomedical imaging and diagnostic platforms increasingly demand stable detection reagents and quantifiable signal output. Upconversion nanoparticles fit the bill and, in the hands of Wenshu Xu and Verity Jackson, allow for multiplex biomarker detection.
Upconversion nanoparticles (UCNP) are a new generation of light-emitting particles with many attractive properties for life science and biomedical applications, including low biotoxicity, excellent photostability and improved quantum efficiencies.
When conjugated to antibodies or oligonucleotides, UCNPs can be used as a label to detect biomarkers both in vitro and in vivo. But until now the limited stability and compatibility of UCNPs with biological systems has often hampered commercial applications.
Recent successes in surface and conjugation chemistry allow us to bridge the gap between UCNPs luminescent properties and their utilisation in biomedical and life science applications, such as high-sensitivity immunodiagnostics, digital pathology, deep tissue imaging, and others besides.
In this blog we discuss:
- Why UCNPs?
- Getting the (surface) chemistry right
- ‘Click’ chemistry
- Commercial outlook
Why UCNPs?
Upconversion nanoparticles (UCNP) are lanthanide-doped metal particles between 1-100 nm in size that absorb multiple low energy photons in the near infrared (NIR) and emit a single photon of higher energy in the visible region.
This so-called anti-Stokes shift in UCNPs can be used to sidestep one of the key weaknesses of traditional fluorophores as well as newer quantum dots: UV excitation causes many molecular structures in biological specimens to auto-fluoresce in the same regions of the spectrum as the emissions of many fluorophores, resulting in pervasive background noise and low signal-to-noise ratios.
But since the NIR radiation that excites UCNPs does not cause biological specimens to auto-fluoresce, UCNP-based systems make it possible to apply high-power NIR and to obtain a strong emission, but without elevating the background noise.
UCNPs are also far more resistant to photobleaching than fluorophores and quantum dots. This offers opportunities to create stable detection systems and, in turn, improved signal quantification – an increasingly important element in life science and diagnostic platforms.
Finally, the absence of noticeable biotoxicity of these nanoparticles, combined with the fact that NIR can penetrate deeper into tissue than UV, means that there is great potential to apply UCNP-based reagents in vivo, for example for whole animal imaging.
Surface coating
Although this is now changing, UCNPs are notorious for being difficult to handle. They can be prone to self-aggregation and falling out of solution, especially under the conditions favoured by most biological molecules.
As a component in detection reagents, UCNPs including their functionalisation must be stable (for long-term storage) and well-dispersed in aqueous buffer systems (for well-characterised reagents with predictable properties).
As a rapidly growing body of literature and patents attests, the “surface coating” of UCNPs can be tuned to achieve this. For example, the surface charge can be tuned to reduce self-aggregation and non-specific interactions with other surfaces, cells and tissues.
Yet, in some environments the UCNP’s coating and surface charge do not completely shield the UCNP. As a consequence, they can still be sensitive to the pH, ionic strength or certain buffer components they are exposed to, causing self-aggregation or non-specific binding.
For example, some buffers commonly used in conjugation reactions or biological assays can be detrimental to the stability of UCNP-based detection systems. We have recently demonstrated that the choice of assay buffers is crucial for achieving a high signal-to-noise ratio in UCNP-based in vitro imaging [1].
‘Click’ chemistry
After tuning the surface coating, for many applications, it is necessary to conjugate biomolecules, such as antibodies or oligonucleotides, to the UCNP’s surface.
“Click” chemistry is emerging as one of the versatile tools deployed for bioconjugation in drug discovery and chemical biology. It is particularly useful for biomolecule conjugation to UCNPs, as it tolerates a wide range of aqueous buffer systems and works with high efficiency under mild reaction conditions.
It also enables the biomolecules to be conjugated to the UCNP in a highly specific and well-controlled manner, which is important for the monodispersity, homogeneity and reproducibility of the final product.
For example, antibodies can readily be modified with a “click”-ready moiety, which reacts with the functional groups on the surface of the UCNP. This highly specific reaction allows the antibody to attach to the UCNP without interfering with its ability to bind its antigen.
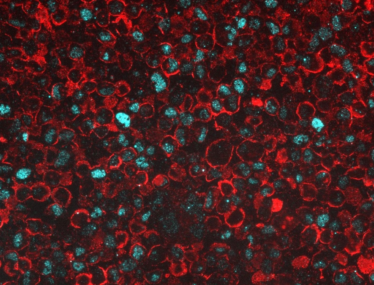
We have just demonstrated the multiplex detection of two clinically relevant biomarkers, one on the cell membrane (red) and another one in the nucleus (cyan), in cells using conjugated UCNP reagents developed at TTP.
Click chemistry also offers the advantage of reduced downstream purification compared to other conjugation methods and are already heavily exploited in the antibody-drug conjugate (ADC) industry.
We will be discussing click chemistry and its application in biology further in a future blog.
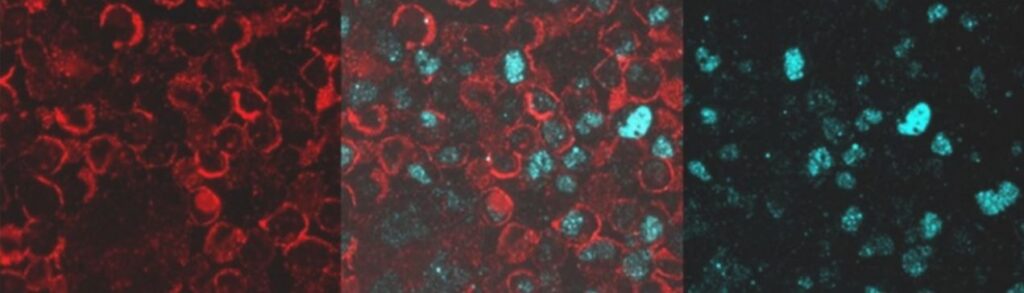
UCNP multiplexed detection of two clinical biomarkers found in cell membrane (red) and cell nucleus (cyan) [2].
Commercial outlook
UCNPs are starting to be exploited commercially in quantifiable tissue immunostaining, high-sensitivity immunoassays and point-of-care diagnostics. UCNP-based products and related instrumentation for both R&D and medical uses are also becoming available.
Demand for a quantifiable and stable signal output in digital pathology and diagnostics make UCNPs an ideal candidate for new detection systems. When there is a clear and strong visual component, this can be combined with deep learning for medical image analysis and used to transform routine tasks into a more automated process, as we have discussed in our previous blog Imaging by the numbers: quantitative imaging for digital pathology.
In addition, progress in terms of reagents and instrumentation for in vitro applications could also provide a stepping stone toward realising UCNPs commercial potential in high contrast and quantifiable in vivo diagnostic imaging.
Diagnostics | Life Science | Medical Imaging | Digital Health | Biosensors | Healthcare Blogs | Healthcare Case Studies