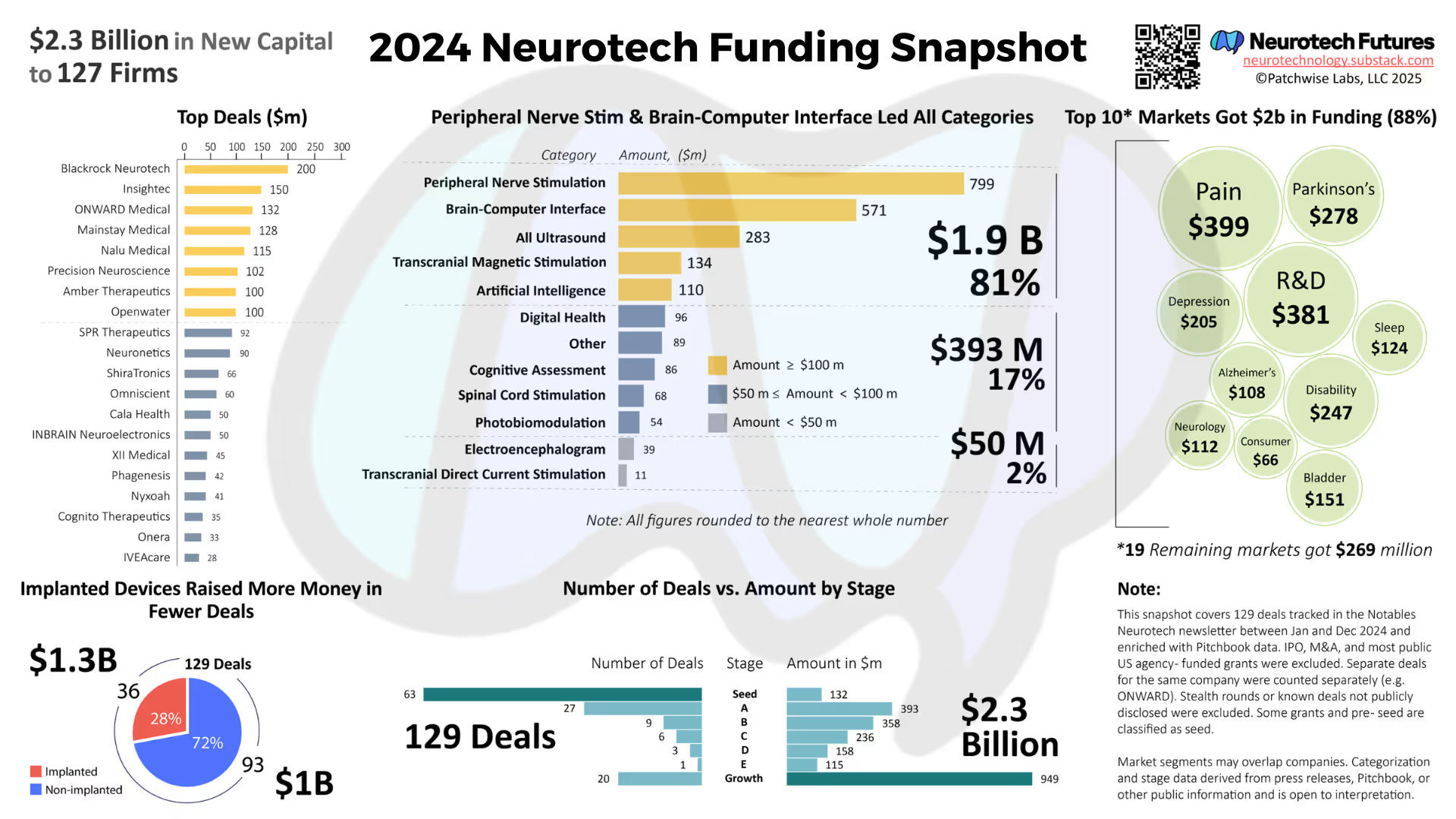
In 2024, Neurotech Futures tallied $562 million in investments into implanted brain-computer interface technology companies. Notable deals included: $200m to Blackrock, $102m to Precision Neuroscience, $50m to INBRAIN, and $18m to Motif.
2025 has already been a record-breaking year, with $856 million invested into BCI in the first half of the year alone, as per HSBC’s H1 2025 report. Led by Neuralink’s eye-watering $650m, deals also included $104m to Science, $50m to Echo, and $17m to Subsense.
Investor interest has expanded beyond traditional medtech capital, with strategic philanthropies, non-profits, and cryptocurrency funds all investing serious money into established or emerging neural interface startups, while many smaller companies enter the field.
To help us explore what it takes to develop a commercially viable BCI, we turned to TTP's Neurotechnology team. Together we identified three areas to investigate:
- Estimated costs required to develop a new BCI, from concept to first-in-human
- Cost breakout across major phases of the R&D lifecycle
- Key trends and pressures shaping the evolution BCI development costs
To better understand the commercial outlook, Neurotech Futures posed these questions to five implanted BCI company founders (Series A through Series D) who agreed to respond anonymously to share their perspectives and insights.
What are baseline cost estimates?
We’ve often heard $100m as a ballpark figure for BCI development costs.
As per Schalk et al, in Translation of Neurotechnologies, 2024: “estimates to develop a new class III medical device, from concept to premarket approval (PMA), are US $94 million.”
But the authors’ cited source describes a separate 2010 survey of 204 medical device manufacturers building “innovative new medical devices” performed by a consultancy, on behalf of a lobbying firm. So not very recent, objective, or specific to neural interface development.
In their 2022 Jama paper, “Estimated Cost of Developing a Therapeutic Complex Medical Device in the US,” Sertkaya et al summarise the overall R&D lifecycle for implantable class III devices.
- $20m for the “nonclinical phase” which encompasses the creation of a proof of concept, development of a device prototype for animal testing, and in some cases, discussions with FDA under one or more Q-submissions, successful completion of the prototype and IDE application and IRB application,
- $32.1m for the clinical phase, which includes conducting a human feasibility study followed by a pivotal study.
While this paper used more up-to-date data from 2018, it is similarly non-specific to brain implants.
So, let’s hear from the real experts: Founding BCI CEOs.
Beyond the literature: founders weigh in
Three of the BCI founders we spoke with argued costs have gone up substantially, especially to run a full clinical program including the pivotal study. Two cited a global cost of $150m-$200m range to prove out BCI with a “high sophistication” in terms of capabilities and intelligence, or “fully implantable systems, with bidirectional recording and stimulation.” A third founder offered a much higher estimate of $400-600m but did not provide further context.
Two BCI founders stated the cost for getting to first in human (FIH) milestones for a “Gen 1 device” have come down to around $30m. They suggested it is possible to achieve FIH to test safety and basic functionality by making use of the “availability of existing, approved devices.”
A key market trend shaping the future is the emergence of specialised next-generation neural interface components via original equipment manufacturers (OEM), such as Mint Neuro’s purpose built integrated circuits, or the growing BCI ecosystem from Science Corp.
At Mount Sinai Biodesign’s 2025 NY BCI Symposium, Science founder and CEO Max Hodak shared that off-the-shelf electronics are “rewriting the economics of BCI development,” shortening time to market and bringing costs down “from >$50 million to <$5 million.”
So: If initial clinical validation costs are coming down, why are competitive, next-gen BCI systems becoming more expensive to develop? To understand this, let’s look more closely at the broader commercial medical device development lifecycle.
Where does the money go?
Schalk et al’s paper describes that of the $94m estimate, “US $75m (80%) is spent on the testing and validation (such as packaging, transport, cleaning, sterilisation, mechanical stability, electrical safety, biological/ toxicological/ histopathological safety and compatibility), fulfilment of standards (for example, ISO 9002) and applications necessary for regulatory approval.”
TTP’s experts provided an overview of how class III development costs typically break out across different phases of the product lifecycle.

The founder feedback we received generally lined up with TTP’s estimates. Several estimated that getting a novel device through pre-clinical testing and submitting to the FDA for an investigational device exemption (IDE) runs around $20m.
Funding the development of a fully implantable electronics system to complete IDE studies, plus the costs of the studies themselves were pinned between $50-$100m, again depending on device complexity.
The additional context provided offers clues into commercial strategy for BCI companies.
- CEOs and CTOs perspectives on such cost breakdowns will differ. The former frame cost breakdowns around key fundraising milestones, while the latter focused on engineering. For investors, this illustrates the appeal of a strong founding team, rather than just a single rockstar founder.
- Preclinical testing can often be done through academic grants that precede incorporation of the company, and sometimes such non-dilutive capital can be used to get a device through the IDE application.
- Across these phases an estimated 75% or more of all funding goes towards engineering and testing, which could also include vendor contracts. One CEO shared that as companies mature, intra-BCI hiring is happening and could further drive up costs (see below.)
- Vertical integration and choice of regulatory pathway (IDE vs 510k) can drive additional spend, e.g. see the FDA’s recently published MDUFA fee schedule.
How will costs evolve over time?
Whatever costs may be for a given BCI system, with technology, markets, and regulations evolving so quickly, change is guaranteed. The founders offered insights into some of the specific market pressures and other shifting sands at play.
Cost increases: driven by testing and talent
In addition to global market trends like inflation and tariffs, founders spoke to specific regulatory and testing costs, as well as growing competition for engineering talent as primary drivers of spending growth in BCI development.
- "BCI in-vivo testing costs are getting higher in the US (CRO costs have inflated a lot, and as a result, there are a number of companies trying to delocalise their testing to other countries)."
- "I expect that costs of creating new tech stacks for implantable BCIs will rise with inflation since the major costs are human hours and animal testing. It’s possible that AI will reduce human hours by a small fraction."
- "I’d look at the auto industry for a comparable figure. We have to build hardware in a highly regulated market. Costs could drop dramatically if AI significantly replaces human labour (doubtful), or if FDA drops some of the animal testing requirements in favour of AI (possible)."
- "BCIs require specialised talent that is hard to find and finite. The more competition for these employees, the more costs will increase for the industry."
- "Costs could become more expensive due to tariffs and inflation, but it’s hard to say if this will negate all the other factors."
Cost decreases: driven by commoditisation and collaboration
Commoditisation of tech and other resources, as well as more sophisticated vendor and OEM capabilities could reduce costs. Some founders pointed to additional factors, from collaboration to regulatory changes, that could hypothetically bring costs down as well:
- "Over time it will certainly become less expensive as economies of scales kick in."
- "BCI hardware development is becoming cheaper as new companies benefit from the learnings and designs of first-generation BCI start-ups, on what the device actually needs / doesn’t need to achieve in terms of performance."
- "Semiconductor hardware and OEM costs are getting lower due to commoditisation, but a lot of suppliers are in Asia, which might become an issue depending on the tariffs situation."
- "BCI development of established tech stacks should become less costly over time. Gen 2 devices will likely be less costly because these platforms are being built to be upgraded over time."
- "Companies developing platforms and/or components that can be adapted and re-used by others. That would drastically reduce the costs. But, this would not apply if each company wants to do vertical integration of the entire tech stack. If many companies require the same components, there will be a higher incentive for these suppliers to develop larger scale manufacturing with a more competitive price."
- "A clearer regulatory landscape will push costs down. First-movers will educate regulatory bodies like the FDA and pave the way for others. Once the FDA starts developing standards and guidelines, this will drastically reduce the amount of time spent by companies trying to define proper testing conditions, clinical endpoints, etc."
The upshot: change is constant
The perspectives highlighted here offer one overarching takeaway for investors and founders: Each BCI system is different, and things are evolving quickly, so your costs will vary.
Today’s benchmark is approximately $150-$200m to fund development of a de novo BCI system, from initial concept through a regulated first-in-human commercial pathway.
As the field evolves, expands, and further globalises, drawing precise comparisons between different interface technologies and the companies building them will become even more challenging - and arguably less relevant - than it is today.
Take Sam Altman’s proposed company Merge Labs, described as a “brain chip company.” Early reporting suggests their approach may involve gene therapy to modify brain cells combined with “an ultrasound device implanted in the head that could detect and modulate activity in the modified cells.”
The breakdown and timeline of building such a complex and hypothetical approach defy comparative analysis to today’s class III cranial implants, but that hasn’t stopped a $250m fundraise against a Neuralink-like valuation of $850m.
As Hannah Claridge, TTP's Head of Neurotechnology puts it: "The BCI field is at an exciting inflection point, progressing rapidly from scientific exploration toward scalable, real-world impact. Developing a clinical-grade BCI is a significant challenge: it requires combining a deep understanding of basic science, clinical engineering, and complex systems integration. Doing this successfully relies on knowing which building blocks can be integrated directly, which need to be customised, and what must be built from scratch."
As we enter a Precambrian explosion of commercial grade technologies emerging in nanomaterials, biohybrid devices, and non-invasive modalities, BCI are rapidly evolving across scientific disciplines, market indications and applications, and economic frameworks.
So, regardless of how much money they raise and where the dollars go, the entrepreneurs navigating the neurotech frontier will need to forge high-trust, collaborative relationships with their investors, employees, end-users, and technical partners.
About TTP's Neurotechnology team
TTP’s Neurotechnology device development team pioneers the next generation of neuromodulation devices, from proof of concept through to manufacturing scale-up. Combining deep scientific insight with user-centred design, our interdisciplinary experts in neuroscience, engineering and human factors turn complex challenges into breakthrough therapies.
We specialise in active implantable devices, low-power sensing, RF and embedded systems, and advanced modelling, delivering solutions that accelerate time to market and enhance patient outcomes. Whether optimising miniaturised architectures or integrating novel materials, we help clients bring bold ideas to life and deliver lasting clinical impact. Find out more.