Subscribe to stay up to date on the most pressing developments in medical device developments - scroll down to sign up.
Weight loss therapies have gone mainstream - but are we designing for the right users?
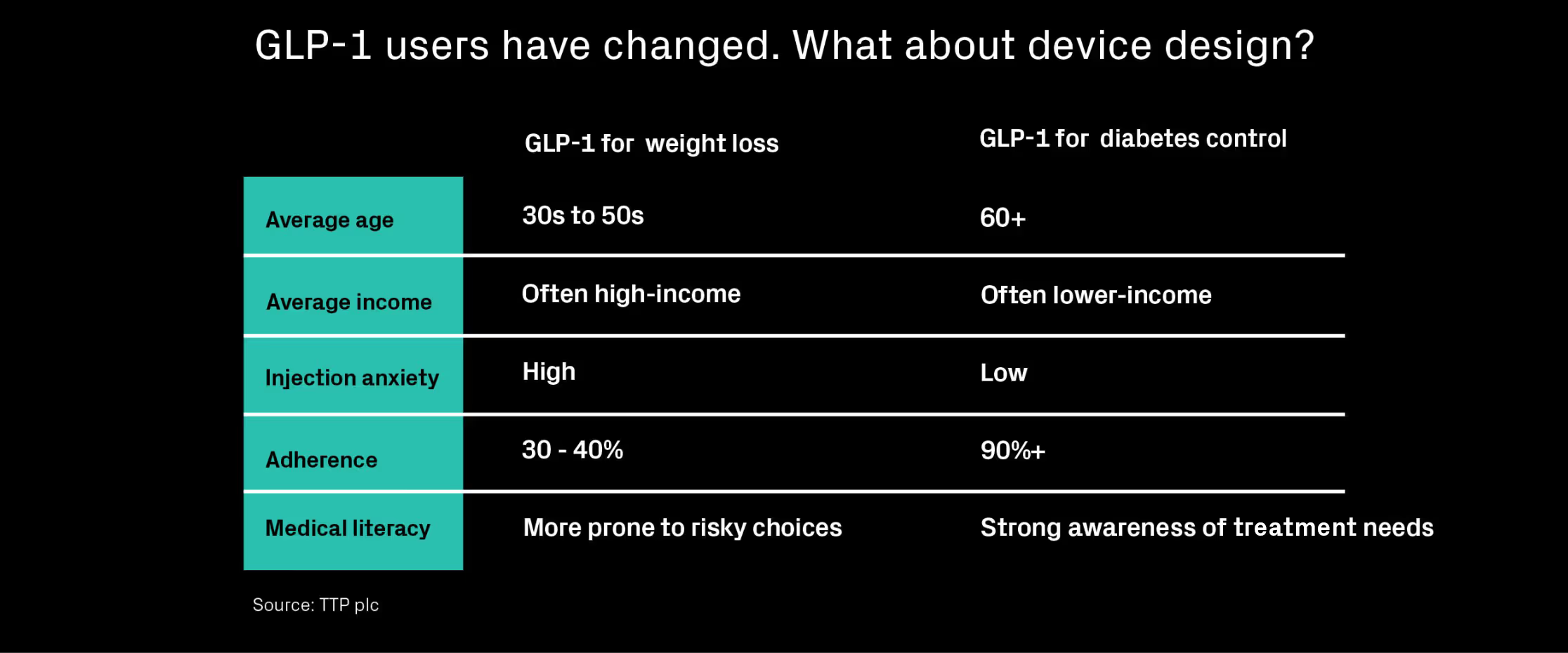
As GLP-1 therapies expand beyond diabetes into weight management, user behaviour is shifting fast. Weekly self-injections, goal-driven motivation, and digital-first health habits all challenge traditional device design. In this piece, we explore how behavioural science and human factors can help teams rethink adherence and usability from the ground up.
Explore the thinking around designing for today’s GLP-1 user.
Why rushing to integrate your prototype could set you back months
In MedTech R&D, early integration can seem like progress, but without validating each module first, it often leads to costly rework.
The smarter move?
Stay curious. Build confidence in every component before committing.
Read First Things First to see how early validation turns integration into a milestone, not a risk.

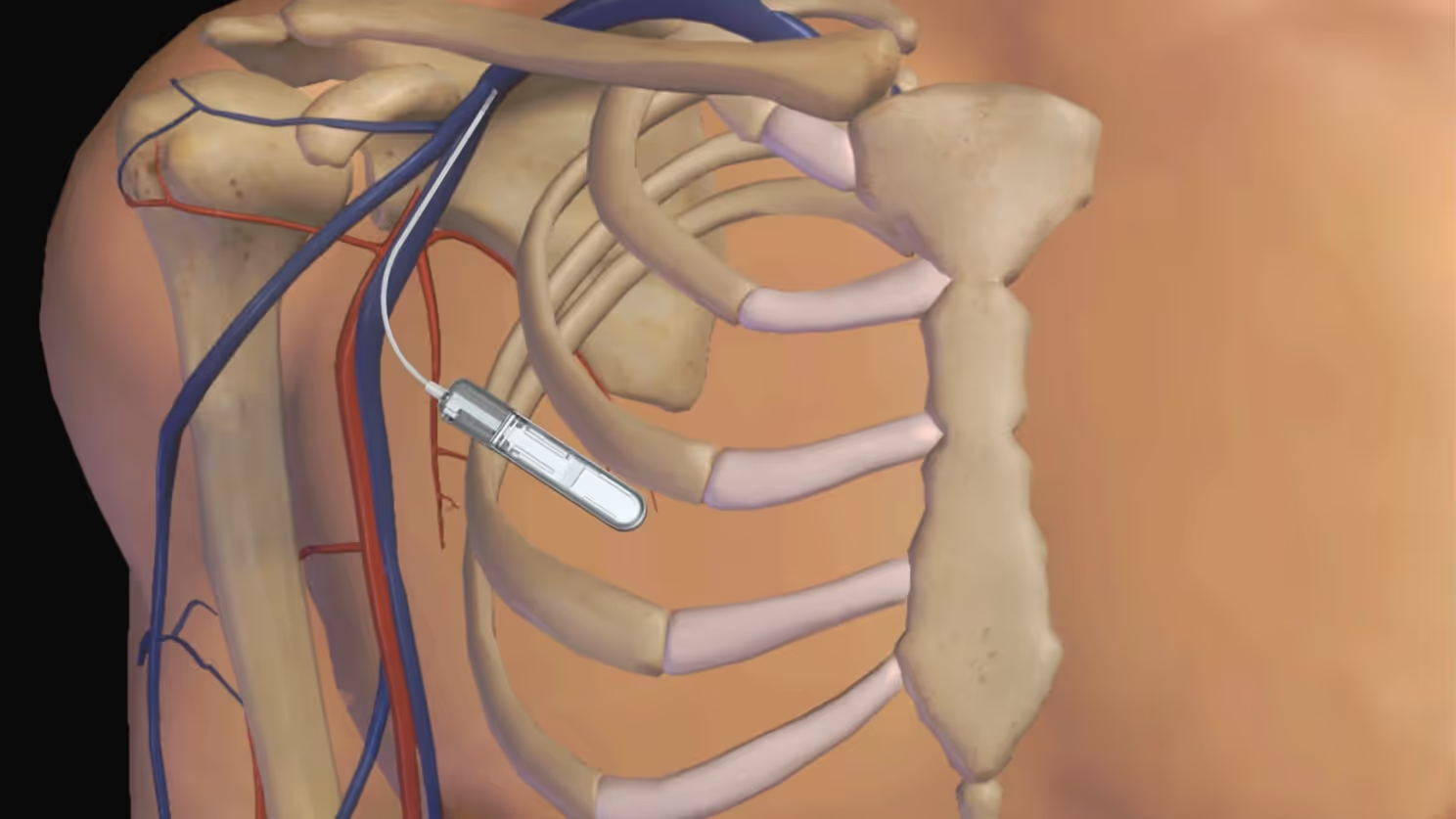
Glucotrack’s breakthrough: 3+ year implantable CGM sensor – without in vivo trials
To avoid years-long studies in predicting sensor lifespan, Glucotrack worked with TTP to model implantable sensor performance in silico.
The result?
A predicted 3+ year lifespan, backed by physical data, and a faster path to market. It helped shape not just the device - but their market strategy.
Read how in-silico modelling forecast long-term performance for Glucotrack.