In this post, we describe how we eschewed complexity – which only adds time to product development – in devising in silico models and a ‘body phantom’ for CGM design optimisation and validation and came up with a reliable test setup for our two candidate antenna designs.
Implantable medical devices are designed in three main stages:
- Devising a ‘body phantom’ system that reliably replicates the important features of a real-world implant–body system
- Modelling the implant to balance conflicting design requirements, such as implant size, battery performance, antenna topology, and signal dissipation (to name just a few)
- Testing candidate designs using this body phantom, to validate them before proceeding with development – to ensure that performance metrics are roughly in line with those expected.
Given the complexity of implant systems, these tasks can seem daunting, and overly complex modelling or test setups add unnecessary time to product development. We use cross-disciplinary systems thinking to overcome these challenges for the development of an injectable continuous glucose monitor (CGM).
Developing the simplest realistic model for implanted antennas
Devising a “wieldy” model of system and antenna performance crucially depends on knowing exactly where and how to make simplifications. Some tissues transmit Bluetooth® frequencies well (such as fat), while others transmit them poorly (such as skin and muscle). What’s more, there is considerable variation in tissue composition within human populations – in our case, in the upper arm where the CGM implant was needed. The implant design needs to work across this variation.
Modelling the passage of RF signals through body tissues is complicated by their varying loss tangent, and so the degree to which the signal dissipates with distance.
Based on previous experience with tissue modelling, we used a three-layer tissue model to capture the essential features of the implant environment and devised a system featuring the following two models to account human variation:
- A ‘general model’ representing the average tissue composition, for assessing typical performance
- A ‘worst-case model’ representing an extreme high-loss scenario, for robustness testing
By addressing both the average and edge case, we were able to ensure that minimum requirements for operational bandwidth and gain are met – so that the antenna maintains robust, user-independent performance in real-world conditions.
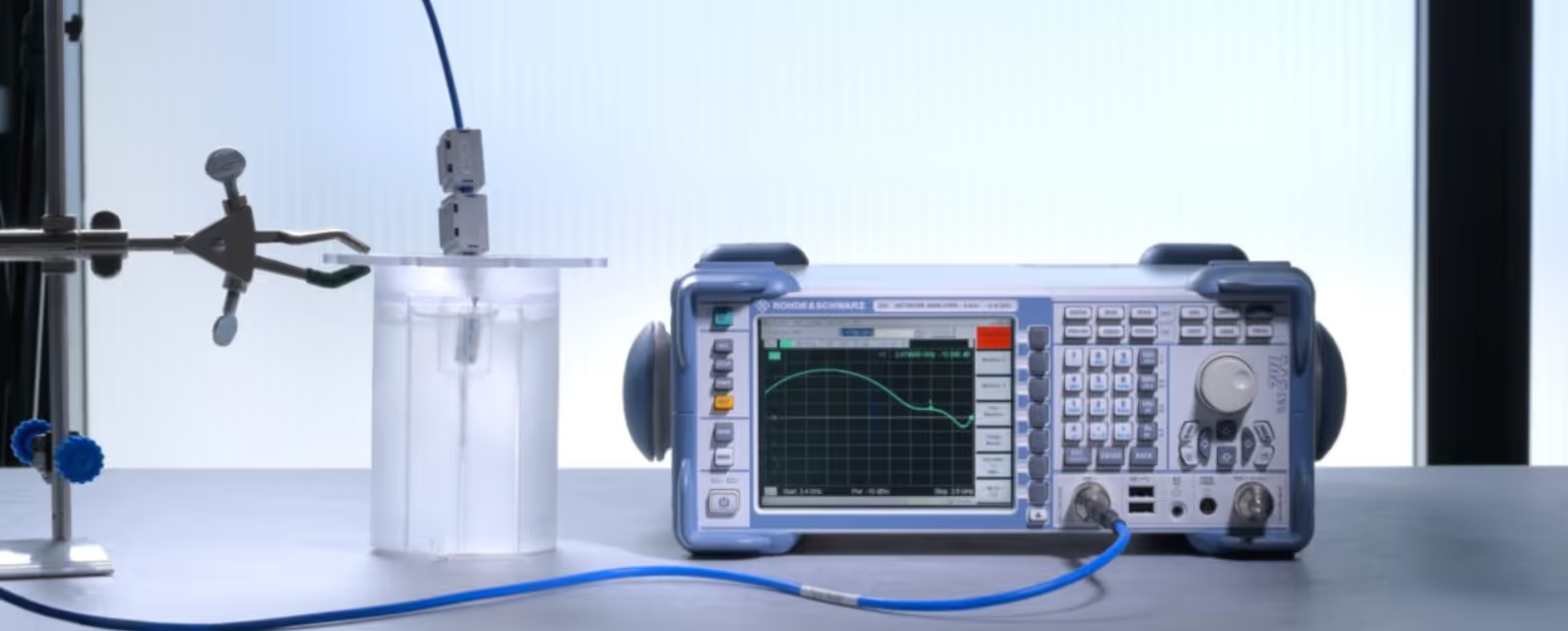
Avoiding unnecessary complexity in the body phantom
We’ve verified our candidate antenna designs by comparing the modelled performance results against those obtained for a prototype device implanted in a ‘body phantom’.
Body phantoms – like human tissues – can be complex, but for assessing signal transmission from a shallow implant, we know it’s sufficient to use a homogeneous single-layer liquid structure. On average, this matches the RF properties of multilayer tissues. Such phantoms have good reproducibility, and from our experience we also knew that they could be easily tuned by adjusting the concentrations of solutes .
Eliminating errors in the test setup
When it finally came to testing our designs, the antenna was fed via a coaxial cable for convenience, with careful cable setup and placement helping to decouple it from the antenna and so avoid measurement errors due to unintended radiation.
An alternative option that eliminates the risk of cable radiation is to use a miniaturised signal generator next to the antenna within the implant. This is appealing because it enables standalone evaluation of compact implantable antennas, but care must be taken to ensure that the intended form factor and electromagnetic performance is maintained.

Design validation: taking a judicious approach to simplification
The development and testing of candidate designs for wireless medical implants is a complex systems optimisation challenge. As we’ve shown in this post, the answer is to take a judicious approach to simplification of both the systems models and body phantoms alongside considering how to eliminate error in the test setup. This enables reliable results to be obtained in a reasonable timeframe, accelerating the time to market.
And at TTP, we’re keen to share our hard-won expertise with you – and make your next biosensing project a success.
Get your copy of the e-book
For more details of how we developed and assessed antennae for an implantable CGM, download the e-Book.











