Cellular Origins partnered with TTP to accelerate the delivery of its Constellation manufacturing platform to the Cell and Gene Therapy Catapult.
Context:
Cellular Origins, a leader in scalable robotic manufacturing for cell and gene therapies, needed to ready and then supply its first integrated Constellation manufacturing platform for a collaboration with the Cell and Gene Therapy Catapult (CGT Catapult). For this time-critical opportunity, Cellular Origins sought a partner they could depend on to accelerate time to launch.
Solution:
Building on TTP’s experience in rapid product design and development for regulated industries, our Advanced Therapies team supported Cellular Origins with their delivery of the Constellation platform to CGT Catapult’s GMP-mirroring Digital and Automation Testbeds – one of the first sandbox environments anywhere in the world specifically designed with automation, digital and robotic technologies for the cell and gene therapy industry.
Result:
One of CGT Catapult’s Digital and Automation Testbeds was equipped with Cellular Origins’ universal automation platform, Constellation. For Cellular Origins, this deployment allows prospective customers to better understand the potential of automation scale-up for their processes and to de-risk the technical transfer to a regulated manufacturing environment. For patients, the impact will be a reduction of therapy cost at scale, and improved access to these life-changing therapies.
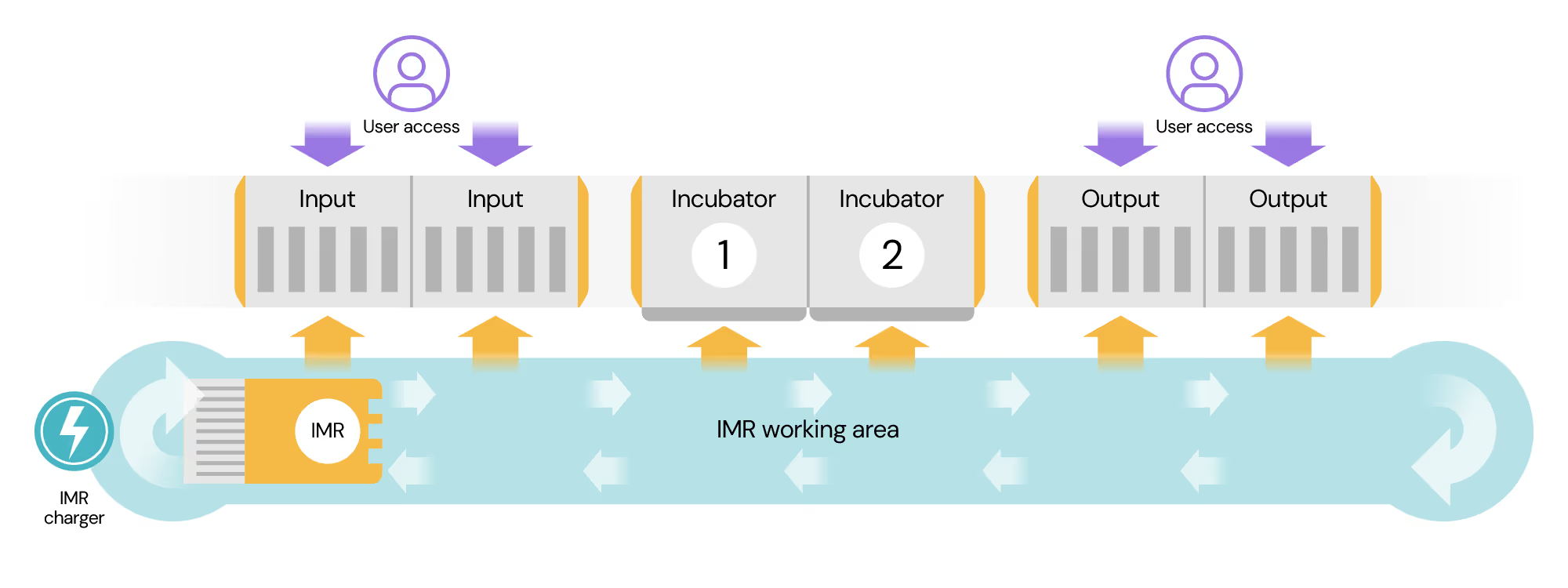
A rapid, collaborative path to first deployment
Cellular Origins is on a mission to enable the manufacture of cell therapies at scale. Its industry-leading Constellation platform uses mobile robotics and AI to automate and connect existing bioprocess unit operations tools employed by cell therapy developers.
Cellular Origins installed a Constellation system into the Catapult’s £2.75M Digital and Automation Testbeds, to deliver on CGT Catapult’s mandate to reduce cost of goods in advanced therapies through driving the adoption of cutting-edge robotic and digital tools. These testbeds mirror the GMP environment necessary for manufacture of cell therapies, and allow therapy developers access to state-of-the-art automation, digital and robotics solutions to support the development of scalable cell therapy manufacturing workflows.
This deployment was an opportunity for Cellular Origins to release to the market, for the first time, several key subsystems within an integrated framework, so that users could make a meaningful assessment of its current and future capabilities. These capabilities included the “work cell” that houses bioprocess equipment and cell culture incubators and Cellular Origins’ autonomous robots. All the work needed to be completed, and the system installed at CGT Catapult’s Stevenage Manufacturing Innovation Centre facility in a timely manner.
When we had the opportunity to deploy our first Constellation platform at the CGT Catapult, we needed a partner with a track record in designing hardware for cell and gene therapy manufacturing, the necessary insight to support robotic integration, and the ability to move at speed alongside our own team. Through our partnership with TTP, cell therapy developers now have access to testbeds to support the development of scalable cell therapy manufacturing workflows.
Dr Edwin stone, CEO, Cellular Origins
In order to capitalise on the opportunity, Cellular Origins needed to accelerate its development and systems integration. TTP’s Advanced Therapies team supported the development and delivery of Constellation into the GMP-mirroring environments at CGT Catapult.
TTP leveraged its insights in launching products into regulated environments, identifying areas where end-user feedback would be most valuable, collecting and consolidating this information into the system architecture for robotic integration. In parallel, TTP’s teams supported Cellular Origins' development activities, and then aided the successful delivery and commissioning of the Constellation cell therapy manufacturing platform at the CGT Catapult.
Shortly after installation of the Constellation manufacturing line, CGT Catapult opened its Testbeds to advanced therapy developers and technology providers, an occasion marked by a visit from Lord Vallance, UK Minister of State for Science, Research and Innovation.
The Constellation system deployment at CGT Catapult was instrumental to establishing a £1M grant-funded consortium between Cellular Origins, CGT Catapult, and Resolution Therapeutics to demonstrate the vision of fully automated, scalable cell therapy manufacturing. It’s an illustration of the impact this system is having in the field, making a real difference in achieving the reduction of costs and increase in manufacturing efficiency and capacity required to bring transformative therapies to patients.













