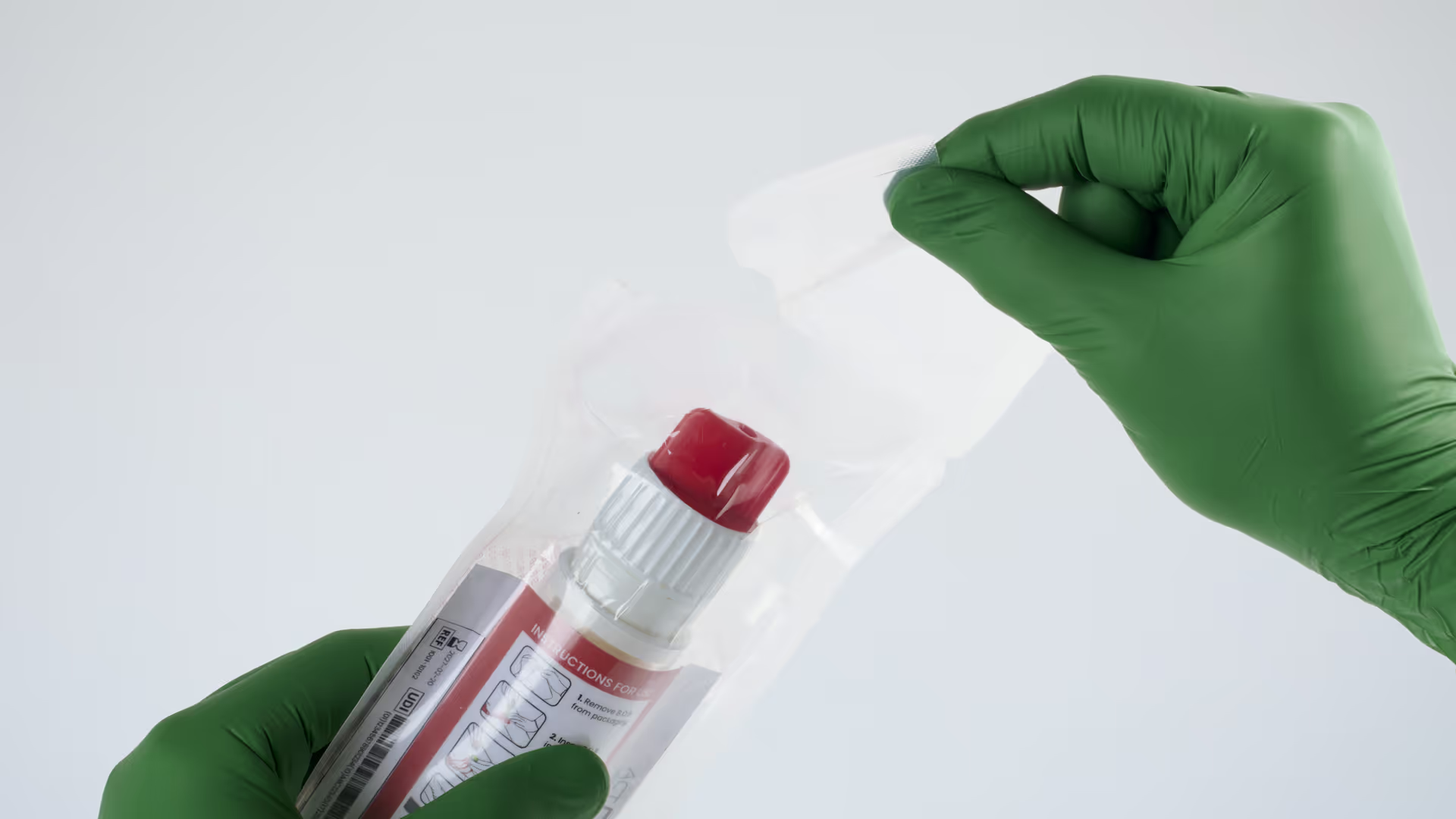
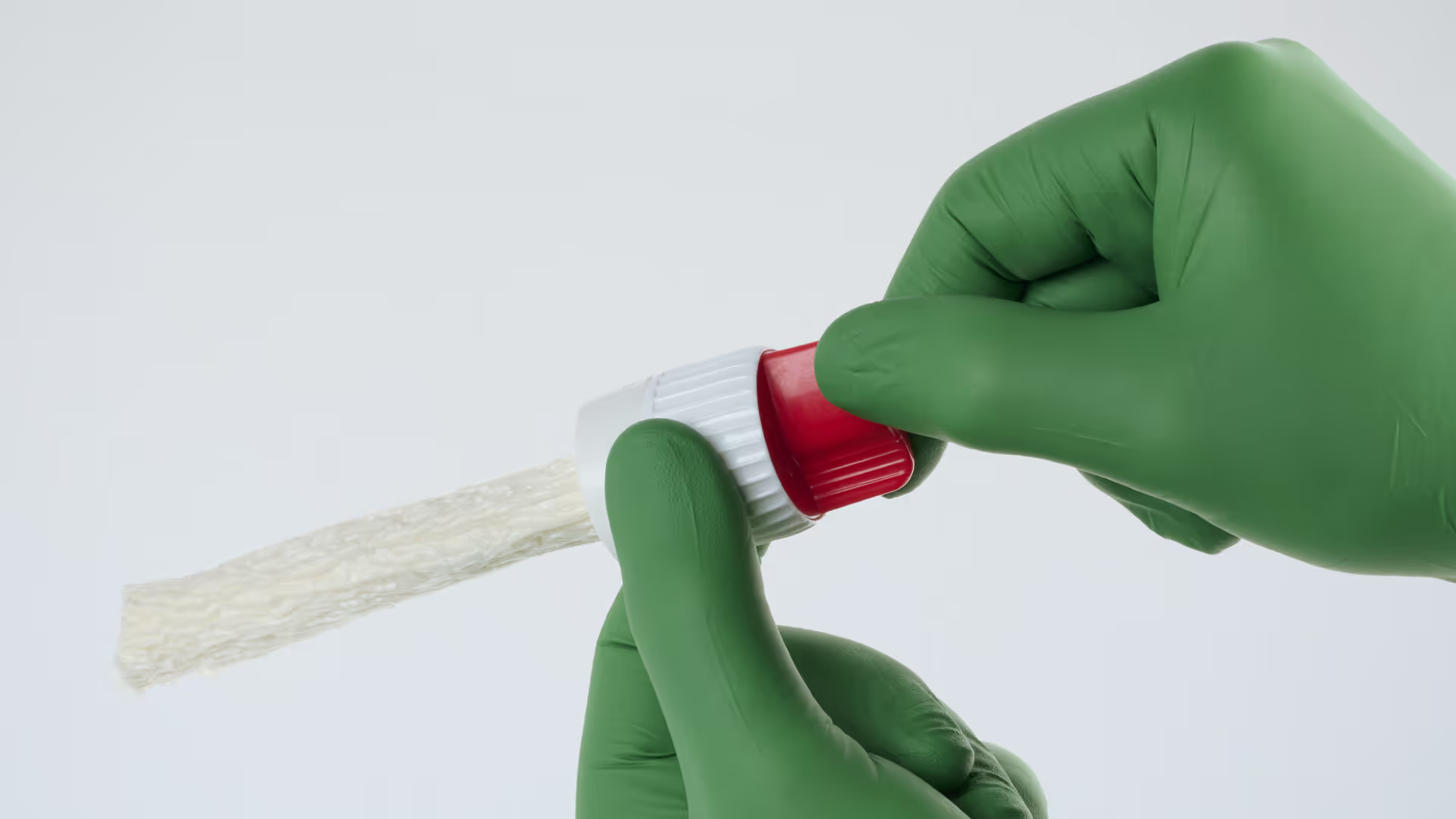
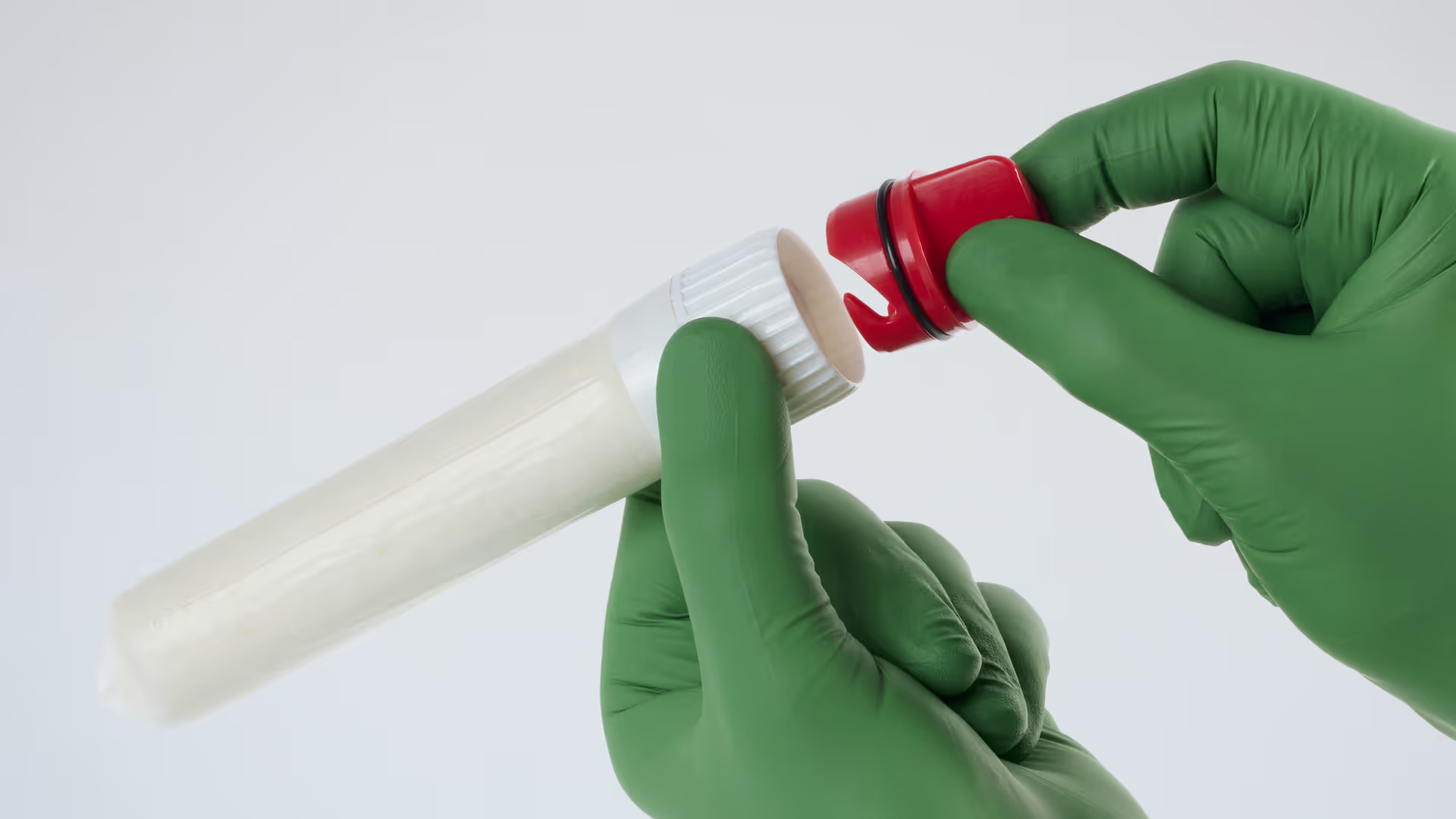
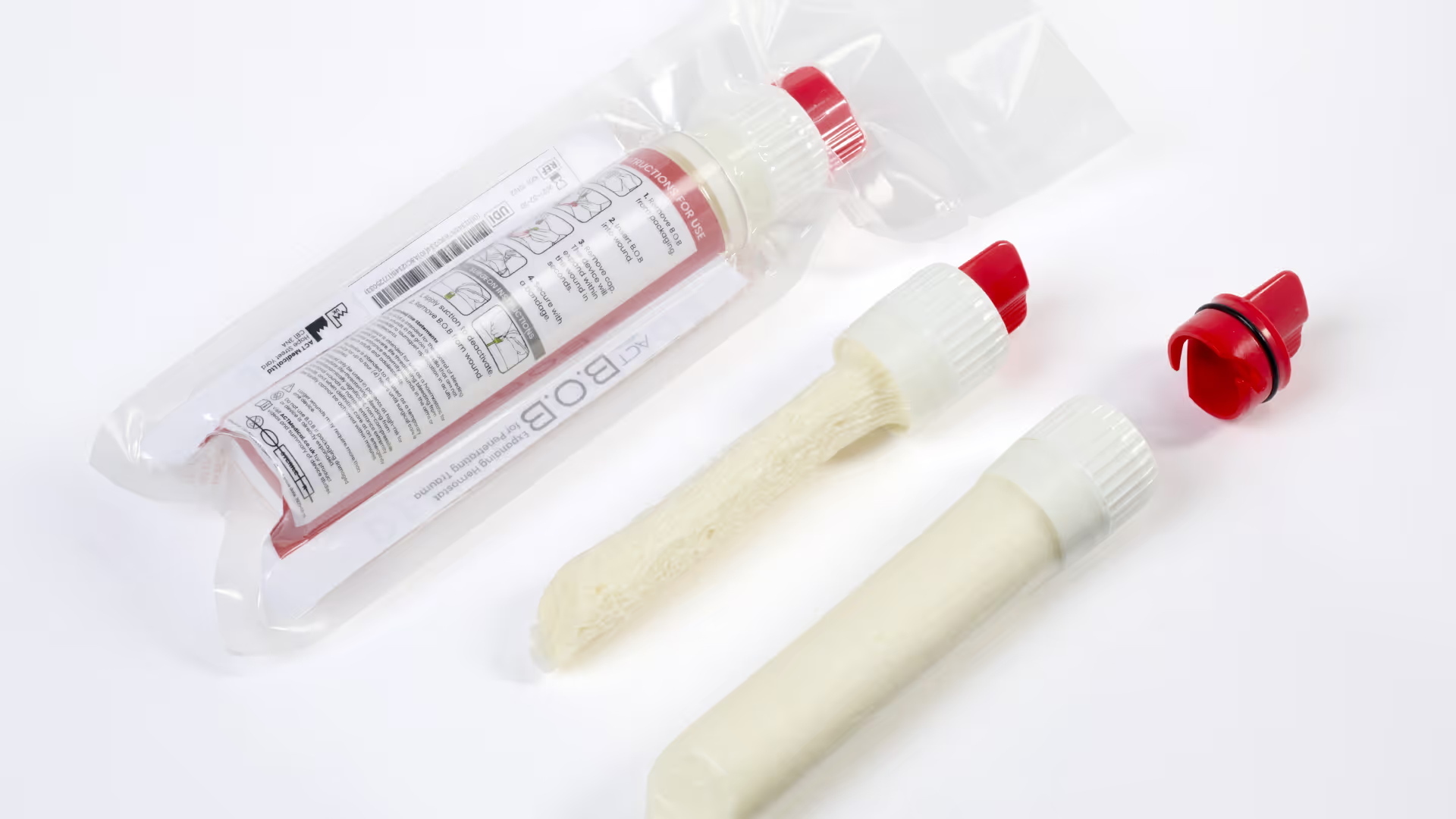
When someone close to you is stabbed and life is at risk, you need to act fast, make brave decisions and get things right first time.
It’s a similar story with our partnership with ACT Medical, where a strong relationship with our client and our approach to medical device development enabled them to create a first-of-its-type medical device for the treatment of stab wounds and penetrating trauma.
ACT call the device B.O.B. We celebrate it as a great case study of trust, collaboration and invention in medical device development.

ACT Medical’s vision
ACT Medical’s story began when their founder, Joe Bentley, was still at university.
The stabbing of two friends prompted him to wonder if he could design a solution to help first responders to treat this kind of injury faster.
An early prototype– in essence a balloon to be inserted and inflated inside the wound to stem bleeding – quickly persuaded market insiders of the urgent need for such a product to reduce deaths from penetrating trauma and won Joe the first-ever Global Medical James Dyson Award.
ACT Medical was founded to turn this idea into a fully-fledged medical device. Research with professional first responders made it clear to the team that in first-response settings simplicity and rapid and effective deployment – literally within seconds – will be key to the success of the device.
ACT Medical approached TTP to contribute to the development of a mature and market-winning solution.
From rapid prototyping to novel concept
During the first stage, in line with our approach to medical device development, our main objective was to rapidly explore a wide range of concepts, with maximum efficiency, to make sure that no promising development direction was missed.
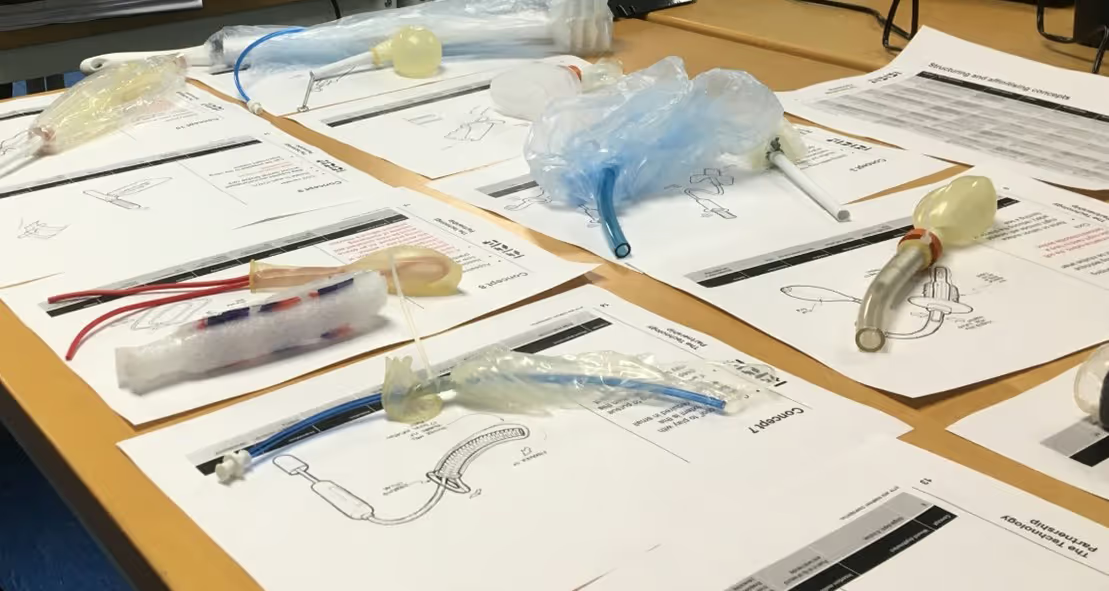
In our own labs, we quickly built more than 40 early concept prototypes, ranging from inflatable balloons to bottlebrushes and auxetic-style mechanisms strapped together to achieve just enough fidelity to carry out quick, hands-on tests in workshops with ACT Medical and evaluate which ones showed promise.
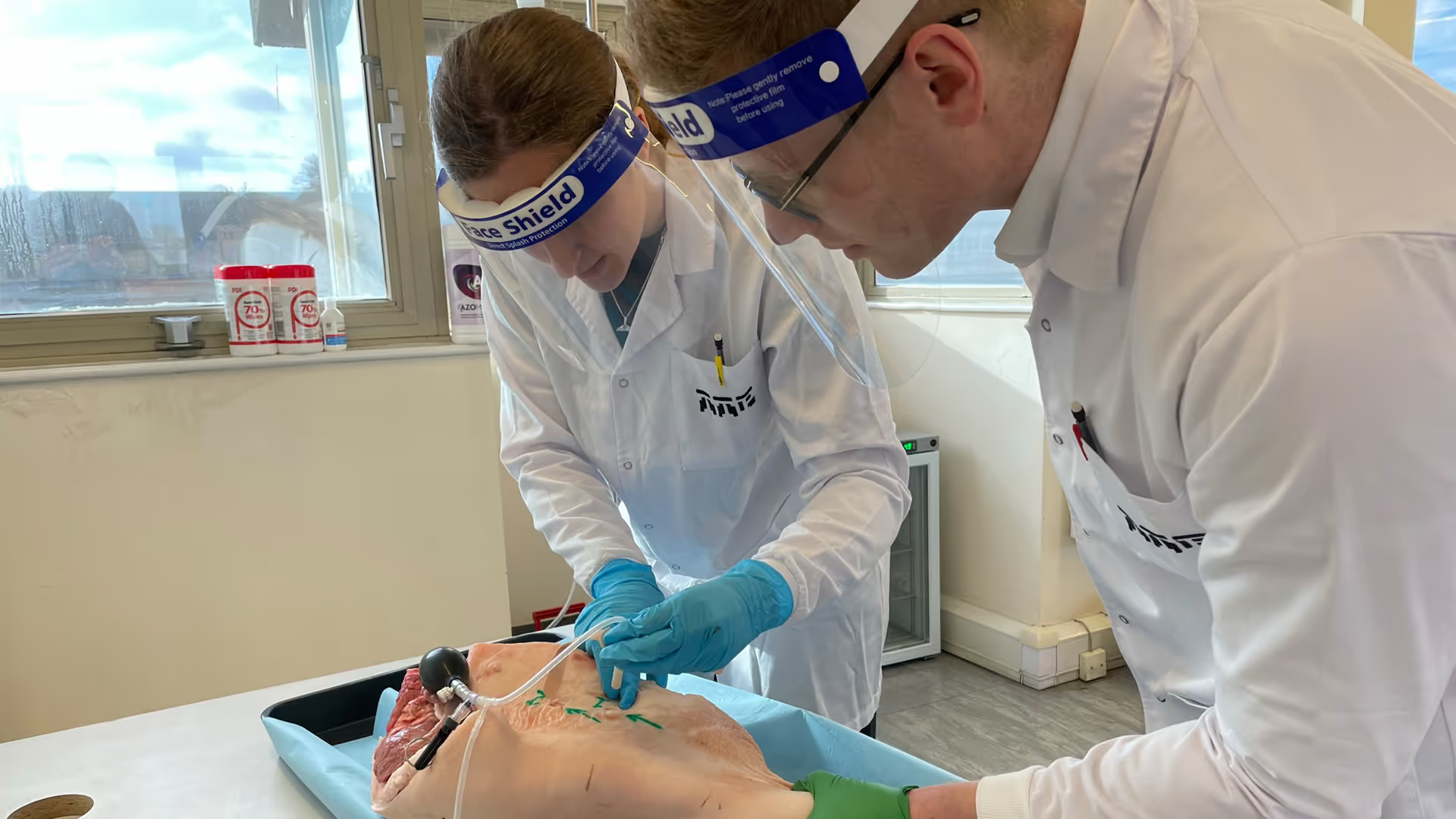
Studies with perfused animal tissue in TTP’s simulation lab taught us much about what may work well intreating real stab wounds. Further user studies with Paramedics and enhanced realism – bleeding limbs prone on the floor and with only non-clinical bystanders to assist – gave us additional insights into how professionals might succeed and fail with such a device in a real-life situation like a mass casualty incident.
A fork in the road for ACT Medical
The first, exploratory stage of our work with ACT Medical yielded two main directions for the development of their device.
The first would lead to a medical device that was simpler to handle and, importantly, more intuitive to use for paramedics – both key for rapid and effective deployment. But it relied on a core component that did not yet exist. Such a requirement typically creates significant additional project risk.
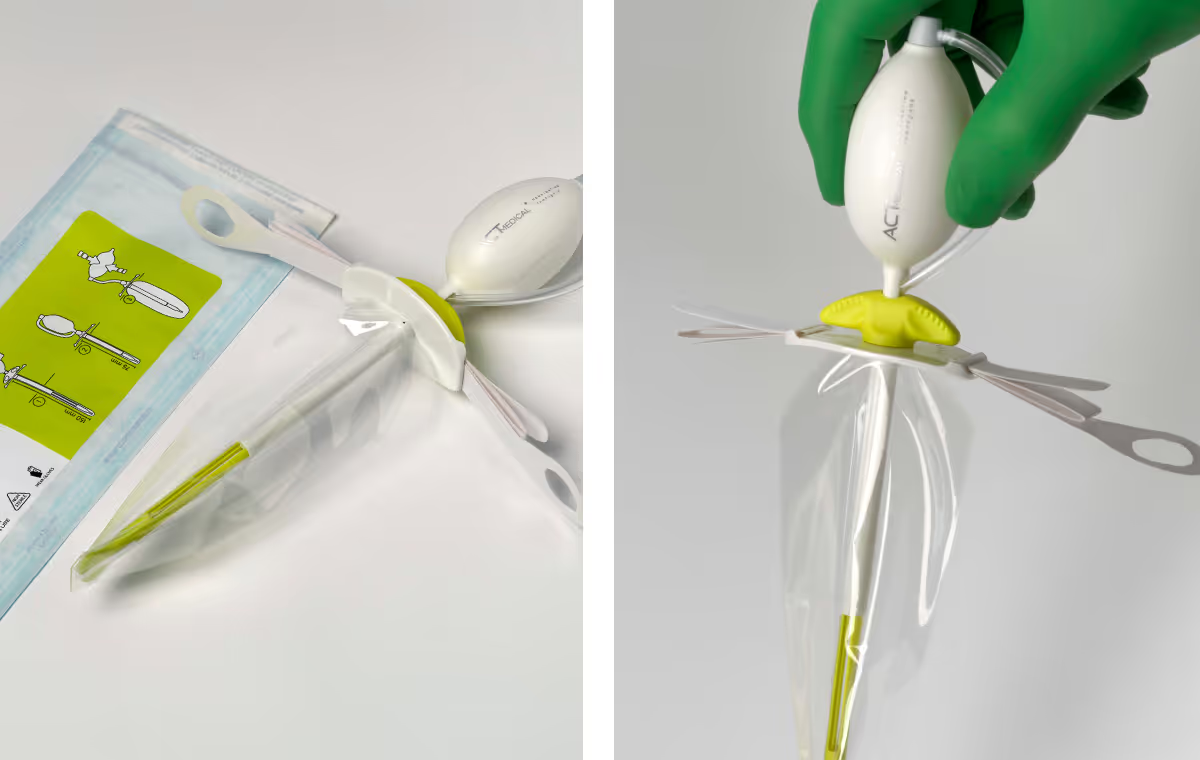
The second was more in line with ACT Medical’s previous thinking and prototypes. It consisted of already-proven components, but it was not as simple to use and - crucially -the way it worked was not as intuitive for first responders.
For early-stage companies like ACT Medical, backing two horses is not an option, so this choice between the best-but-high-risk or lower-risk-but-less-effective development path would prove pivotal.
Mitigating risks to support a confident decision
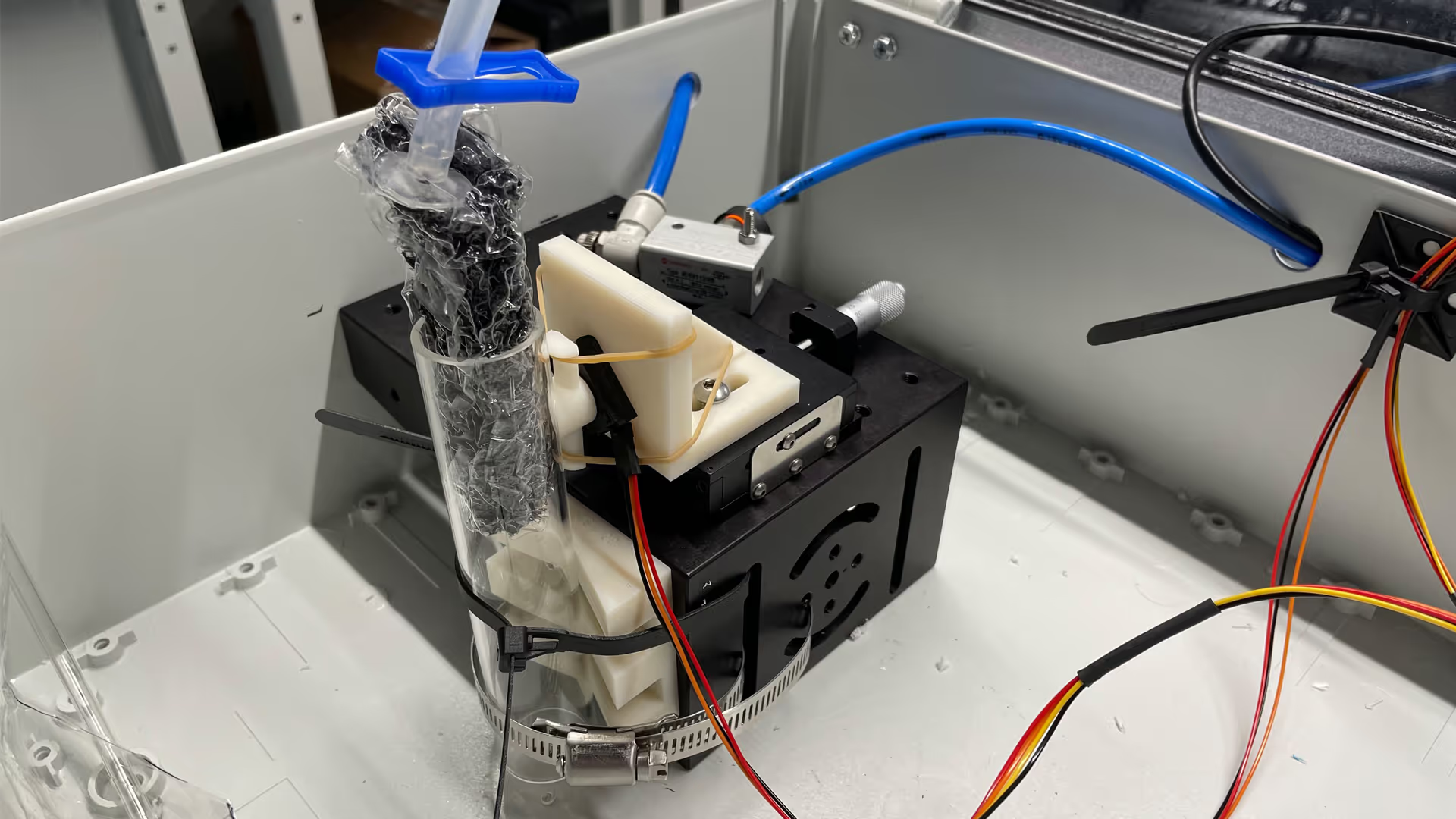
We quantified and mitigated the development risks for ACT Medical by defining the properties that would be needed for the novel core component and identifying a possible manufacturer.
Building on our deep understanding of tissue architecture and perfusion, we modelled the structural and mechanical parameters that the component would need to have to stop the bleed. We then generated the necessary IP to enable ACT Medical to exploit this direction, and identified a possible manufacturer with the specialist know-how to prove the core component was feasible.
When our development with TTP reached the point of a critical decision, they provided us with information and created the necessary space to weigh the risk and reward ratio of both options, which ultimately allowed us to make the decision to develop B.O.B.
Joe Bentley, ACT Medical
With the technical risk quantified, and our early exploratory work giving confidence that there wasn’t another low-hanging solution, ACT Medical decided to pursue the first development direction – of a first-of-its-kind device with novel core component for the treatment of penetrating trauma. ACT named this new device B.O.B.
Minimum-viable product demonstrates ease of use and effectively stops bleeding
ACT Medical progressed the development to a minimum viable product (MVP) to demonstrate the intended final form and functionality with an intuitive two-step workflow: inserting the device and removing the cap to activate it within the wound.
TTP supported a second user study with U.S.-based first responders, which surfaced finer details around user interpretation, feedback cues, and communication requirements for clear instructions for use. Importantly, even untrained participants deployed the device quickly and effectively, validating the intuitive nature of its mechanism and design.
Meanwhile, ACT Medical completed an in vivo pre-clinical study, demonstrating that direct internal pressure reliably stopped bleeding from junctional wounds within three minutes in 100% of cases, with no adverse effects observed.