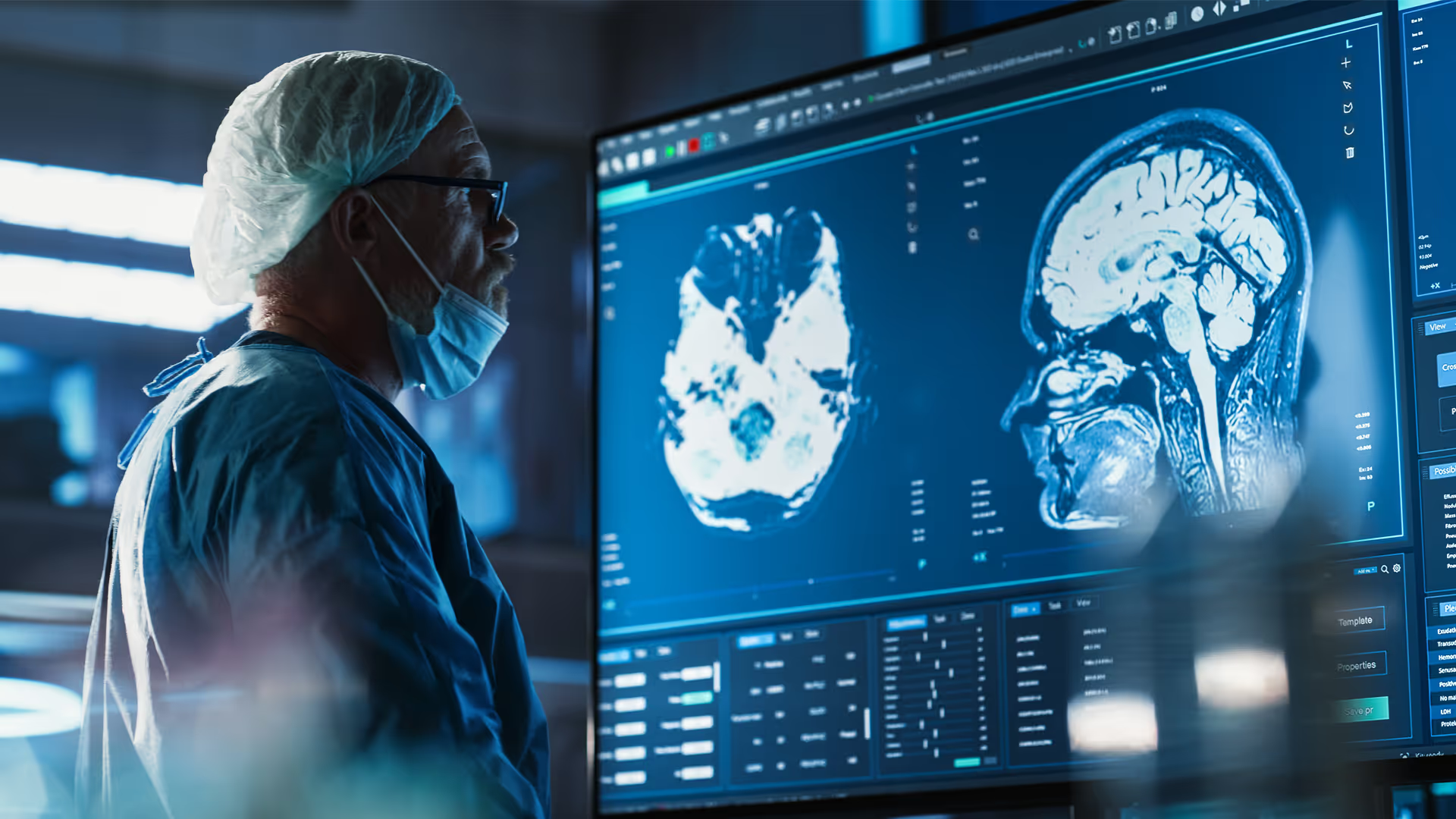
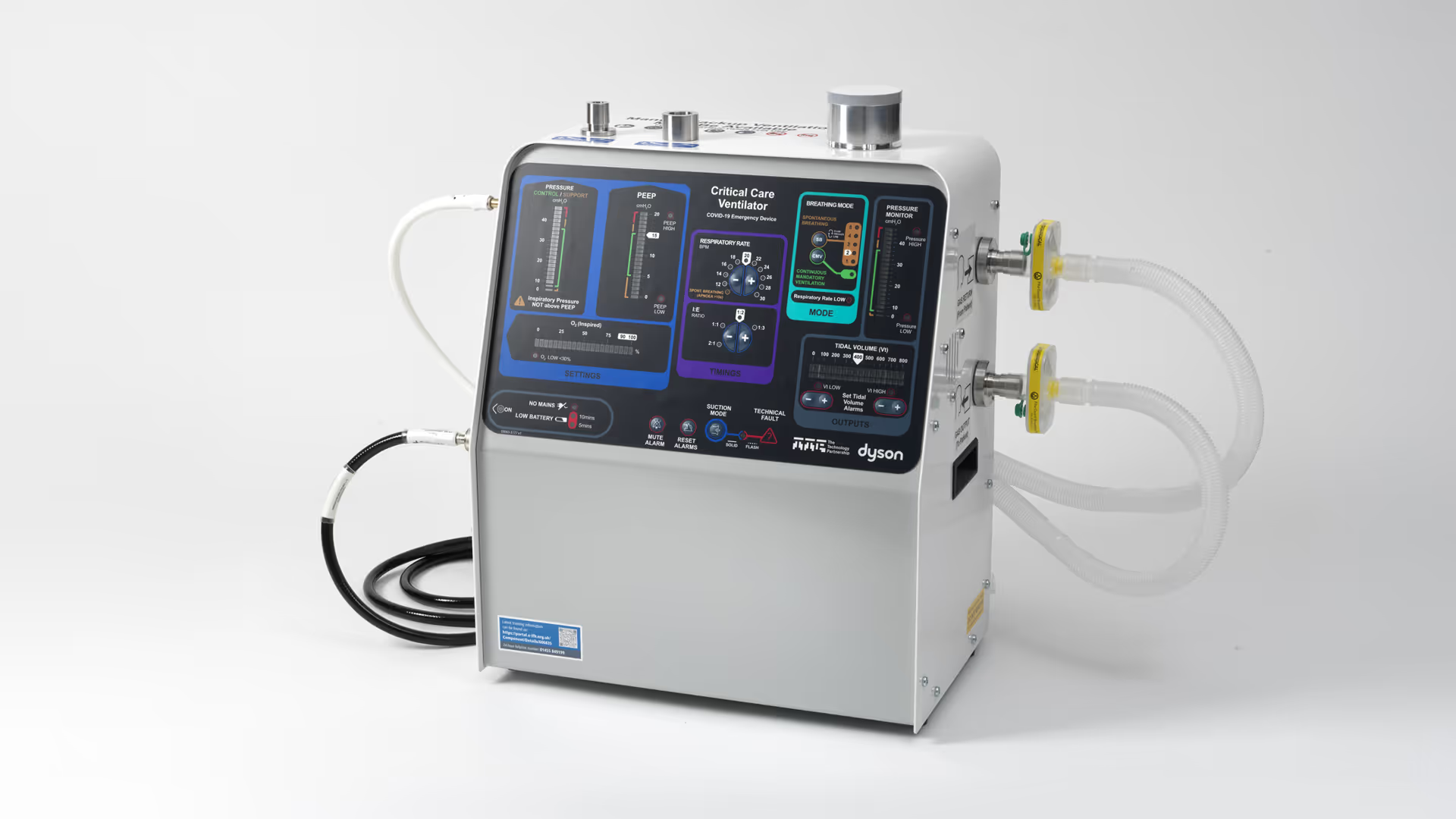
Cardiovascular device development
Solving the toughest challenges in cardiovascular innovation.
With decades of experience advancing medical technologies, we help innovators design, develop, and commercialise the next generation of cardiovascular devices. Our multidisciplinary teams combine engineering, science, and human factors expertise with capabilities in robotic-assisted interventions, advanced imaging, algorithms, and digital modelling to tackle complexity and accelerate innovation. From structural heart to rhythm management, we deliver technologies that improve patient outcomes, meet clinical needs, and achieve commercial success.


Structural heart interventions
Designing technologies for structural heart interventions requires navigating complex anatomical, physiological, and regulatory challenges.
At TTP, we apply first-principles thinking, advanced modelling and systems engineering for next-generation solutions - delivering safe, reliable, and high-performing devices across prosthetic valves, repair systems and occlusion technologies for our clients.
We work closely with clients at the forefront of transcatheter intervention, supporting the development of robotic-assisted systems, fusion imaging platforms, and smart implants with embedded sensing and connectivity.
These technologies are enabling greater standardisation of complex procedures, reducing clinician cognitive burden, and improving procedural precision and the consistency of clinical outcomes – while also supporting shorter learning curves and new models of training and supervision, such as teleproctoring.

TTP has been creative, thorough and quick. We now have a much better solution to meet the needs of our unique operational environment.
Dwight Meglan
Chief Technology Officer
,
Heartlander Surgical

I’ve had the opportunity to work closely with TTP over the past 20 years, focused on solving very diverse and complex engineering challenges, and required to deliver breakthrough product technology to the market. They’ve been incredible partners and fully integrated members of our R&D team and have shared tremendous passion, energy, and drive to succeed to help support our physicians and their patients.
Joshua Stopek
VP: R&D
,
HistoSonics Inc

We gave TTP an underdefined, complex therapy characterisation challenge in a novel therapy space. They, in turn, provided a systematic discovery plan, delivered rigorous and understandable modelling and neuroscience outputs, and ultimately steered us towards a workable concept for a new-to-the-world therapy concept. I was reliably impressed with their responsiveness, the quality of the outputs, and their ability to iterate at high velocity towards answers to really hard questions.
Steven Goetz
VP Technology & Platform Innovation
,
Medtronic Neurom.
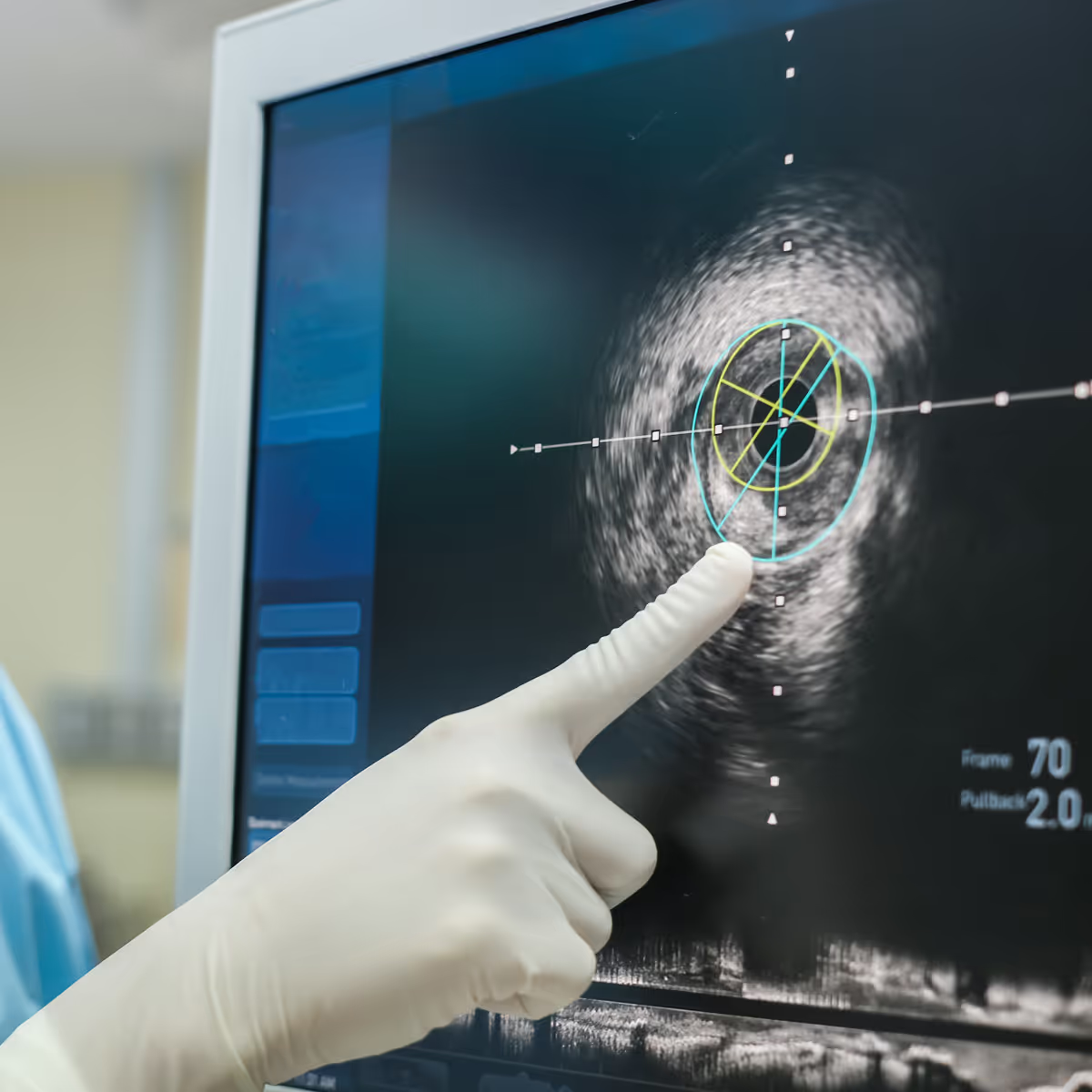

Peripheral and neurovascular interventions
From peripheral artery disease to acute ischemic stroke, TTP supports the development of endovascular devices that restore blood flow and improve patient recovery. Our expertise spans fluidic management systems, stent and implant delivery platforms, thrombectomy and embolic protection devices, and innovative catheter-based solutions.
We integrate fusion imaging and navigation technologies to improve precision across cath lab and neuro interventional or hybrid operating environments. Looking ahead, we are enabling hybrid approaches that integrate mechanical intervention with non-implant based energy therapies, reducing procedure time and improving long-term outcomes.


Cardiac rhythm management and pacing
Pacemakers, defibrillators, and emerging leadless systems demand miniaturisation, power efficiency, and uncompromising reliability. We help clients advance these systems with low-power electronics, wireless telemetry, smart algorithms and biocompatible materials.
In the electrophysiology lab, we support the development of technologies that integrate algorithms, simulations, and digital twin models to optimise device performance and guide therapy.
We also explore remote monitoring solutions that extend the value of pacing and rhythm devices beyond the procedure, enabling clinicians to track patient health and device function continuously.

Ablation and energy-based therapies
Energy-based therapies, from RF and ultrasound to cryo and pulse field ablation, are rapidly advancing as safe and effective treatments for arrhythmias.
TTP brings deep expertise in energy delivery, thermal and electrical modelling, and catheter design.
Our teams are driving innovation in non-implant based energy therapies, supported by fusion imaging, robotic navigation, and real-time sensing to guide precise ablation.
By combining these with remote intervention and teleproctoring capabilities, we help create systems that deliver safer, more effective therapies and expand access to care worldwide.


Collaboration that drives results
Partner with us to advance cardiovascular care. At TTP, we combine scientific insight, engineering rigour, and commercial awareness to support every stage of device development - from initial feasibility through to clinical batch manufacturing in line with ISO 13485 and transfer to high-volume manufacture - helping partners create devices that are robust, scalable, and ready for the future of cardiovascular care. Speak to a cardiovascular device development expert today.
How we can help
Regulatory expertise in medical device development
We help ensure your device development aligns with the relevant standards, preparing the robust design history files required for regulatory submission. Our ISO 13485-certified quality system and deep technical expertise streamline development and reduce friction in later stages.

Human factors expertise for user-centric device development
Our multidisciplinary teams combine human factors experts and engineering expertise to design devices that meet both user needs and technical requirements.
Behavioural psychologists provide unique insights, supported by our hospital-simulated and in-home testing facilities, to capture real-world use cases and refine user experiences. This approach helps avoid common user challenges, delivering devices that enhance patient outcomes.

Computational modelling
To accelerate the product development process, we apply our deep expertise in biomechanical and electrophysiological modelling to explore wide parameter spaces efficiently. This significantly reduces the number of experiments required to make critical design decisions, and the quantitative outputs provide valuable insights into sensitivity of the therapy to manufacturing tolerances, differences in physiology, and surgical placement.

Enhancing surgical navigation and tool tracking
TTP brings deep expertise across disruptive navigation technologies, including proprietary EM navigation, EIT mapping systems, and MR headsets. Leveraging our robust theoretical modelling and in-house R&D prototyping labs, we develop navigation solutions that are not only highly functional but also adaptable to evolving surgical needs, enabling rapid adoption and customisation.
For procedures like total knee arthroplasty orendoscopic navigation, knowing exactly where a tool is - and where it’s going -matters. We develop embedded sensors that help determine tool trajectory and orientation, ensuring precise and repeatable outcomes in complex surgical workflows.

Surgical safety and critical structure avoidance
Navigating complex access routes in minimally invasive surgery is crucial to avoiding unintended damage to critical structures like nerves and major blood vessels. TTP is pioneering a range of technologies for critical structure avoidance. Our advanced, tool-tip sensing solutions provide real-time feedback in a format that is intuitive and actionable, empowering clinicians to proceed with confidence, knowing they’re working safely and accurately.
Whether locating retained surgical items or identifying unexpected anatomical features, we support novel detection capabilities through custom sensing strategies - from miniaturised optical sensors to flexible EIS-enabled microelectrodes - designed for harsh or constrained surgical environments.

Leads and electrodes
Delivery of a new therapy often requires the design of custom leads and electrodes for optimal performance, safety and stability. The interaction between the electrodes and the biological target is impacted by multiple factors including electrode material, electrode shape, and stimulation waveform. With expertise in both traditional leads and thin film electrodes, we transform core technologies into fully-developed, market-ready products.

Wireless power and communications
Active implanted medical devices are not fit and forget; they need to communicate with external devices to set up therapy parameters, enable data collection, and run software updates. Many devices also contain batteries that need to be regularly recharged; this presents a particular challenge as the desire for rapid charging must be achieved within the regulatory SAR limits while minimising any interference with neural activity. We have developed a robust workflow for the design of wireless power and communications systems which don’t incorporate any proprietary IP.

ISO13485 batch manufacturing
Contract manufacturing organisations prioritise long-term, high-volume contracts and often lack the flexibility to support smaller orders. Recognising the challenges in securing low-volume manufacturing for clinical studies, we established a certified manufacturing facility to support our clients and bridge the gap between R&D and full-scale manufacturing. Our bespoke facility provides agile, small-scale manufacturing solutions ensuring are liable supply of devices for clinical trials.

Regulatory expertise in medical device development
We help ensure your device development aligns with the relevant standards, preparing the robust design history files required for regulatory submission. Our ISO 13485-certified quality system and deep technical expertise streamline development and reduce friction in later stages.

Human factors expertise for user-centric device development
Our multidisciplinary teams combine human factors experts and engineering expertise to design devices that meet both user needs and technical requirements.
Behavioural psychologists provide unique insights, supported by our hospital-simulated and in-home testing facilities, to capture real-world use cases and refine user experiences. This approach helps avoid common user challenges, delivering devices that enhance patient outcomes.

Computational modelling
To accelerate the product development process, we apply our deep expertise in biomechanical and electrophysiological modelling to explore wide parameter spaces efficiently. This significantly reduces the number of experiments required to make critical design decisions, and the quantitative outputs provide valuable insights into sensitivity of the therapy to manufacturing tolerances, differences in physiology, and surgical placement.

Enhancing surgical navigation and tool tracking
TTP brings deep expertise across disruptive navigation technologies, including proprietary EM navigation, EIT mapping systems, and MR headsets. Leveraging our robust theoretical modelling and in-house R&D prototyping labs, we develop navigation solutions that are not only highly functional but also adaptable to evolving surgical needs, enabling rapid adoption and customisation.
For procedures like total knee arthroplasty orendoscopic navigation, knowing exactly where a tool is - and where it’s going -matters. We develop embedded sensors that help determine tool trajectory and orientation, ensuring precise and repeatable outcomes in complex surgical workflows.

Surgical safety and critical structure avoidance
Navigating complex access routes in minimally invasive surgery is crucial to avoiding unintended damage to critical structures like nerves and major blood vessels. TTP is pioneering a range of technologies for critical structure avoidance. Our advanced, tool-tip sensing solutions provide real-time feedback in a format that is intuitive and actionable, empowering clinicians to proceed with confidence, knowing they’re working safely and accurately.
Whether locating retained surgical items or identifying unexpected anatomical features, we support novel detection capabilities through custom sensing strategies - from miniaturised optical sensors to flexible EIS-enabled microelectrodes - designed for harsh or constrained surgical environments.

Leads and electrodes
Delivery of a new therapy often requires the design of custom leads and electrodes for optimal performance, safety and stability. The interaction between the electrodes and the biological target is impacted by multiple factors including electrode material, electrode shape, and stimulation waveform. With expertise in both traditional leads and thin film electrodes, we transform core technologies into fully-developed, market-ready products.

Wireless power and communications
Active implanted medical devices are not fit and forget; they need to communicate with external devices to set up therapy parameters, enable data collection, and run software updates. Many devices also contain batteries that need to be regularly recharged; this presents a particular challenge as the desire for rapid charging must be achieved within the regulatory SAR limits while minimising any interference with neural activity. We have developed a robust workflow for the design of wireless power and communications systems which don’t incorporate any proprietary IP.

ISO13485 batch manufacturing
Contract manufacturing organisations prioritise long-term, high-volume contracts and often lack the flexibility to support smaller orders. Recognising the challenges in securing low-volume manufacturing for clinical studies, we established a certified manufacturing facility to support our clients and bridge the gap between R&D and full-scale manufacturing. Our bespoke facility provides agile, small-scale manufacturing solutions ensuring are liable supply of devices for clinical trials.

Our approach and capabilities
We deliver across the entire life of a project, from opportunity discovery to production engineering. Discover how our interdisciplinary teams of experts collaborate to tackle the toughest product development challenges.

Our campus and facilities
Our award-winning campus has been designed with a clear vision. To create a space which can support our people and our clients as we develop and deliver the very best technology solutions.

Meet some of the team

Hannah Claridge

Paul Galluzzo

Marco Grasso

Talk to us about your next project
We help clients with all stages of their most complex and challenging technology and product development projects.
If you're considering the next steps along your innovation journey, why not get in touch?