Cell therapies are now being considered for ever-larger patient populations and a more diverse range of indications. Each cell type and cell modification technique undergoes significant process development effort to ensure desired performance and quality attributes – with each cell processing workflow being custom to the company or group developing it. Scaling needs are often not fully satisfied by off-the-shelf bioprocessing solutions, leaving many cell therapy developers exposed without clear routes to scale up and commercialisation.
Clearly, novel manufacturing systems and timely availability of technologies will be needed to truly seize the commercial opportunity that comes with addressing disease areas with such high unmet need. But timing is everything, and validation of new hardware at late clinical stage can take up precious bandwidth when cell therapy developers find themselves stretched to deliver therapies for clinical trials. This risks extending timelines to launch.
Given these competing demands, when is the right time to think about the manufacturing scale-up strategy for your cell therapy? To answer this, it’s worth revisiting “Concurrent Engineering”, a tried and tested approach in engineering established and proven in many industries.
What is Concurrent Engineering?
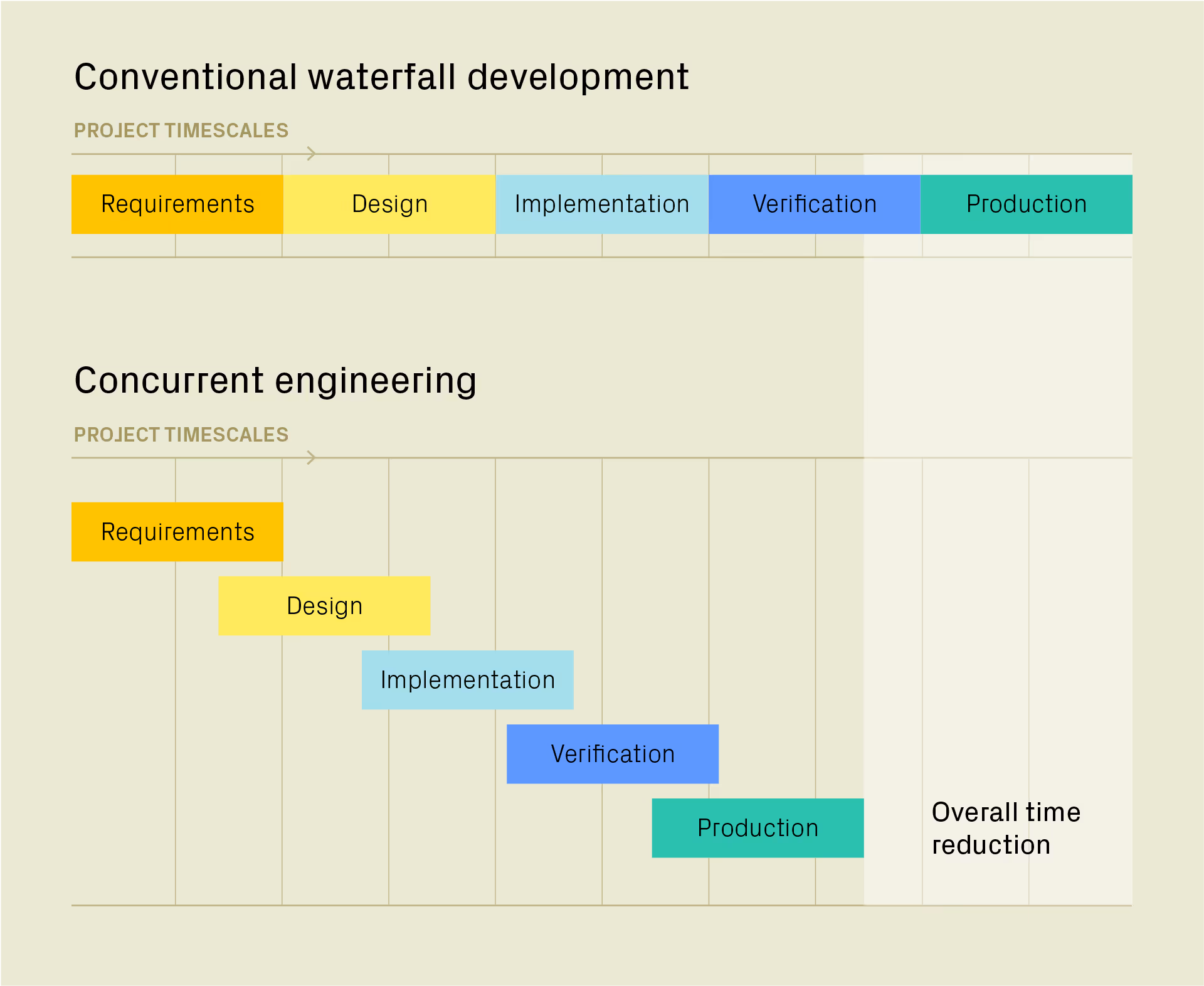
For high-risk, high-value products with a high cost of deployment, traditional engineering development typically follows a “waterfall” or stage-gated approach.
Engineering challenges and phases of work are tackled one after another – for example, concept generation, feasibility, detailed design and scale up for manufacture.
For developers, this offers clear checkpoints and control. As work during any stage builds on the firm basis of all the results of the previous stage, the risk of rework is greatly reduced. But it also comes at the cost of speed, because there is no opportunity to make progress until the results of the previous stage are in.
In concurrent engineering, the workstreams are run in parallel or staggered parallel with heavy overlap of different workstreams. Cross-functional, multidisciplinary teams work together with real-time communications. A collaborative environment ensures that all perspectives are considered from the outset, enabling teams to identify and pre-emptively solve potential issues, optimize processes, mitigate risks and accelerate decision-making – avoiding bottlenecks later on.
Originally popularised in manufacturing industries such as aerospace and automotive design, concurrent engineering came into its own in our organisation during the Covid pandemic, when the patient need was really pressing. As Covid cases were growing exponentially, TTP developed a lung ventilator within six weeks by carrying out all the development activities in parallel. With a stage-gated approach, the same development could have taken 2-3 years. In more normal times, we take the same approach in product development across many industries.
The benefits of concurrent engineering are clear: products can reach the market faster, with wider cross-functional input to cover all aspects of the design, including manufacturability. This also reduces the risk that those manufacturing-critical questions aren’t asked until it is too late to change the process.
Why should cell therapy developers adopt this mindset?
Cell therapy development, with pre-clinical development followed by multiple phases of clinical trials, strongly resembles a traditional stage-gated process, with FDA (or EMA) approval acting as a defining gate.
In traditional pharma, there is no harm in adopting a stage-gated mindset because dedicated Tech Transfer and MSAT (Manufacturing, Science and Technology) can readily transition to scaled-up manufacturing equipment, after late-stage trials confirm the drug’s efficacy.
But cell therapies are different.
Here, depending on the process, the manufacturing equipment is not always available as off-the-shelf products. Some steps in the process may not have a clear route to scale, necessitating customised manufacturing systems which need to be developed, not bought. For companies in late-stage clinical trials with a bigger team, considering scale-up at this stage can cost far more, which poses a real risk of running out of funding.
There is an even more critical reason why you should adopt a concurrent engineering mindset: When it comes to cell therapies, the product is, in effect, defined by the manufacturing process you submit for regulatory approval. If this process doesn’t enable manufacturing at scale, then there is a good chance that you will find yourself without the runway to demonstrate process scalability, as process changes after approval can take months or years.
What does concurrent engineering look like in cell therapy?
For cell therapy developers, the concurrent engineering approach means thinking about manufacturing solutions earlier than a stage-gated approach might suggest.
What’s more, any early investment in refining process steps with scalability in mind will quickly pay dividends. For example, process closure allows a lower grade of cleanroom to be utilised, while enabling parallel processing of patient batches. Both lead to much lower equipment and facility costs from the outset, which is particularly important if a company is planning on building their own facility.
Similarly, targeted intervention to introduce automation sufficient to standardise process steps can reduce variability, which often stands in the way of generating preclinical data, ultimately saving time and cost in executing process development. If these sources of variability and manufacturing cost are eliminated at an early stage, then scale-up presents much less of a risk.
So, the critical question is whether your product can be made reliably and at scale. Concurrent engineering is a way to minimise the risk that cell therapies fall at the hurdle of manufacturability and scalability. And given the relatively long timescales of therapy development, this approach can be implemented without requiring excessive bandwidth as you prepare for clinical trials.
Using Concurrent Engineering, TTP can rapidly develop manufacturing equipment for GMP use tailored for your specific process, improving standardisation and streamlining process development, while avoiding bottlenecks in technology transfer caused by mismatched off the shelf solutions.










