The pioneers of advanced therapies were able to demonstrate market potential by answering the scientific question: can these therapies work? But the market has shifted. Venture capitalists and strategic partners are seeking assets that have been de-risked to a greater extent, including a credible, data-driven path to commercialisation.
What’s more, in advanced therapies it’s often said that “the process is the product”. Every aspect of the process directly affects the therapy’s quality, safety, and efficacy. Once a product/process has been validated clinically, modifying it becomes costly, complex, and time-consuming, sometimes even triggering regulatory review.
At the same time, pharma now bring existing facilities, automation platforms, and production infrastructure. They are not looking to acquire technologies that can only function within bespoke or manual systems. Instead, they want assets that can integrate seamlessly into established platforms.
Despite understanding these issues, many developers grapple with a paradox – as they reach for clinical proof-of-concept, often by open, manual processes for expediency and familiarity, how can they avoid locking in inefficiencies that will drive up the cost of goods and constrain scalability?
How can early-stage companies, often still iterating on their process, make informed decisions about its commercial manufacturing strategy?
The power of modelling cost of goods
The solution, even for processes still under development, lies in a modelling framework that brings together both process dynamics and cost structure to guide decision-making from early development through scale-up.
Such a model goes beyond simple cost accounting.
Modelling COGs starts with a detailed breakdown of the workflow (often over a hundred distinct steps). Using benchmark data for time, labour, and equipment, models can simulate different configurations such as open versus closed systems, centralised versus integrated incubation, or varying levels of automation. This in turn allows developers to test different production scenarios, assess the impact of automation or facility changes, and identify where constraints will emerge as volumes grow.
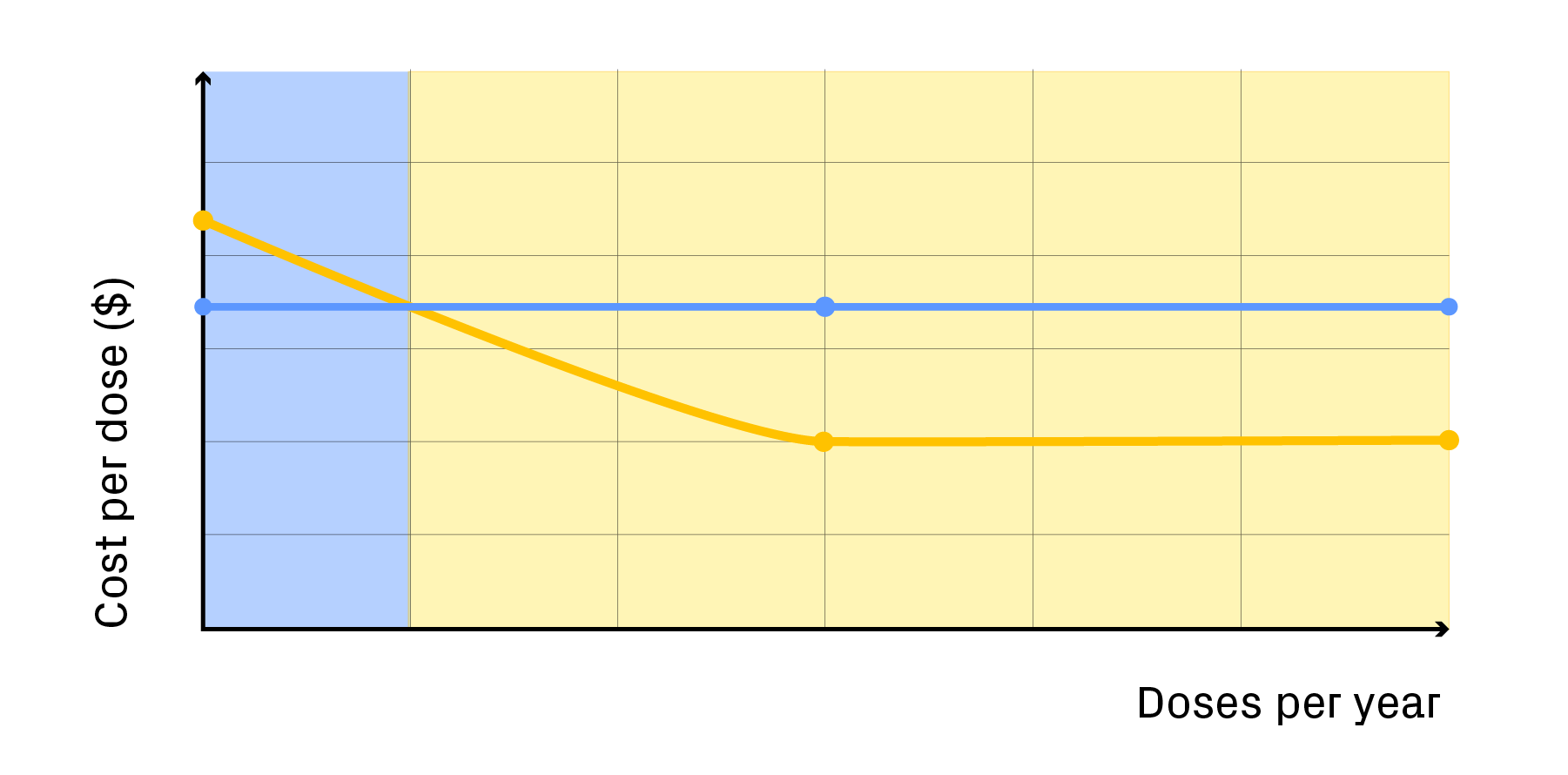
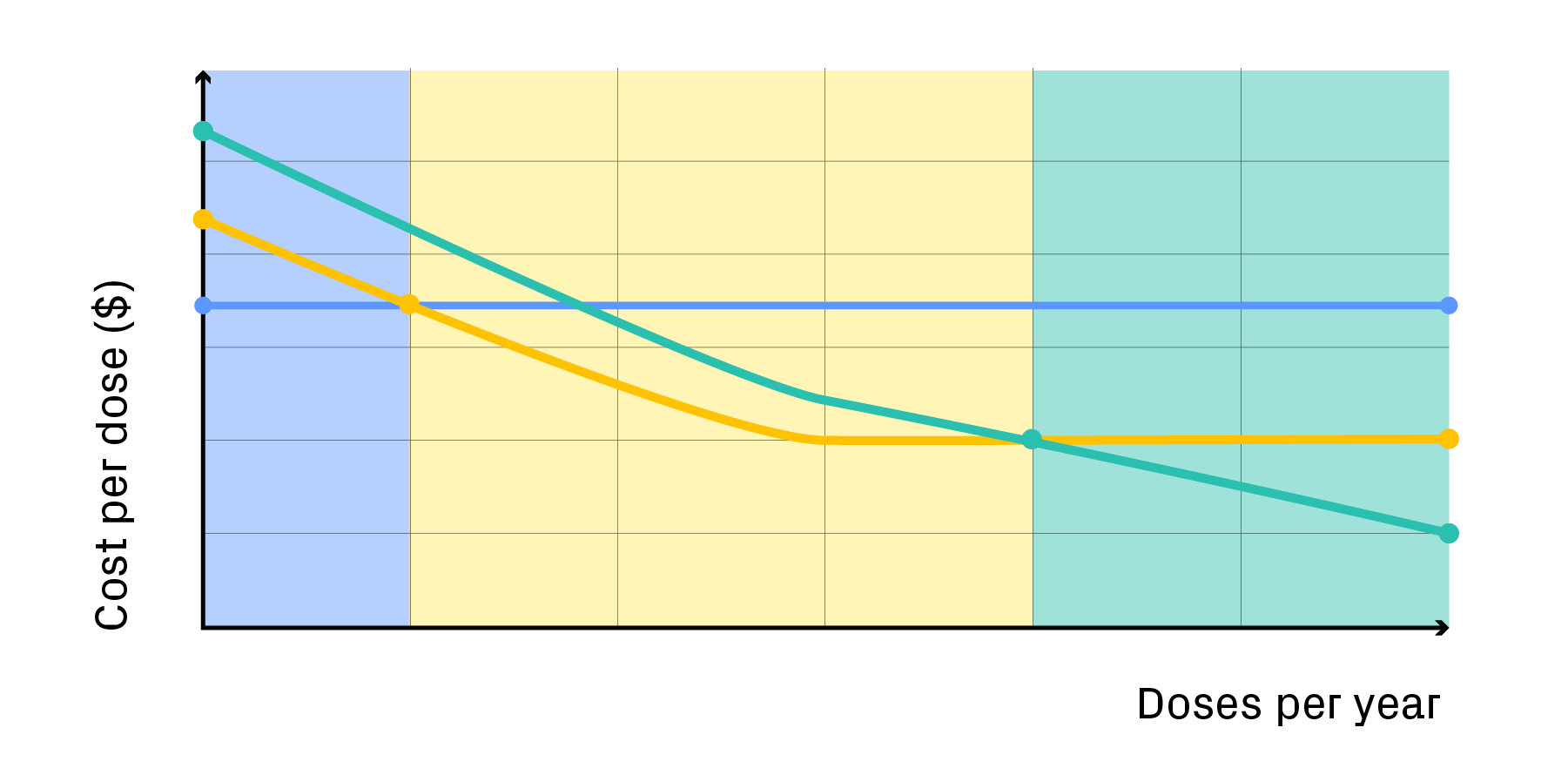
The result is a model that doesn’t just calculate cost per dose but maps out the path to scalability and economic viability, revealing where targeted investment will deliver the greatest long-term return.
Rather than relying on assumptions or industry heuristics, modelling COGs provides tangible data that helps companies and investors make better decisions. It turns questions into financial and operational insights.
How does modelling COGs add strategic value?
1. Assess commercial viability at scale
Modelling COGs quantifies the cost dynamics of a therapy by projecting cost per dose at clinical and commercial scales, identifying break-even points, and pinpointing where automation or process optimisation delivers the greatest return.
For example, a cost model grounded in process may show that automating one upstream step cuts manual labour hours by 30% but increases equipment costs by 10%, enabling data-driven trade-offs between capital investment and long-term savings.
By integrating process flow and capacity constraints, modelling reveals hidden bottlenecks, such as quality control (QC) steps that become rate-limiting as production scales. This visibility gives developers the insight to plan and prioritise changes before they become costly or disruptive.
As one constraint is resolved, modelling helps predict where the next will appear, providing a roadmap for automation and process optimisation to navigate these pressure points. The result is higher throughput, improved scalability, and greater cost efficiency to keep manufacturing strategies aligned with commercial goals.
2. Inform scale-up and operational planning
Once commercial feasibility is established, modelling also supports scale-up planning by translating economic insights into operational requirements. It forecasts operator numbers, shift patterns, facility layout, and equipment utilisation.
For example, recruiting and training skilled operators has been identified as a limiting factor in CGT manufacturing readiness. Modelling helps quantify these needs early, highlighting whether a process demands 10 operators or 100, and whether existing facilities can support that workforce. In practice, these insights frequently demonstrate that automating QC or other manual tasks is not just a cost decision but an operational necessity.
3. Evaluate the impact of technology choices
Every technology choice, from bioreactor design to cell-isolation methods, carries downstream implications. Modelling captures how technology choices affect cost, space utilisation, facility requirements, and scalability simultaneously. For example, transitioning from an open to a closed bioreactor system dramatically reduces cleanroom requirements and streamlines workflows, but may also drive changes to upstream and downstream process steps.
By quantifying trade-offs, cost modelling enables developers to compare alternative technologies objectively, select the most cost-effective configuration, and plan technology adoption in line with both manufacturing and commercial objectives.
4. Identify and prioritise technology aps
Perhaps most critically, augmenting the cost model with an appreciation of the technology landscape highlights where current technologies are unable to support the desired scale or process efficiency. Relying on automation solutions that are available and deployable ‘off the shelf’ can lead to months of process adaptation and comparability testing, at a time when a company’s cash runway is sensitive to even the shortest delay.
Insights from TTP’s cost modelling have enabled organisations to target innovation or partnerships in the right areas and to address issues proactively, rather than reactively during late-stage development. We have identified process steps that pose operational risks, through being labour-intensive, variable or hard to scale. We then work with our partners to develop equipment that supports immediate needs and future scalability.
Talk to us about modelling the cost of your goods. Biotech leaders know that cost is not a simple number; it’s a web of interdependent variables. At TTP, our approach to modelling COGs goes beyond financial analysis. We bring together expertise in process engineering, automation, and commercial strategy to build models that reflect real-world manufacturing dynamics. Where technology gaps are identified on the manufacturing roadmap, we work with our partners to identify and, if necessary, develop solutions. In this way, we help biotech leaders quickly uncover where value will be gained or lost, so you can make the right investments now, avoid costly redesigns later, and scale with confidence when it matters most.











