Vagus Nerve Stimulation (VNS) is a well-established therapy for epilepsy, but today it’s typically delivered continuously, which can limit effectiveness and cause unwanted side effects. TTP's Neurotechnology team explored whether seizure activity could be detected directly on the vagus nerve itself.
Context:
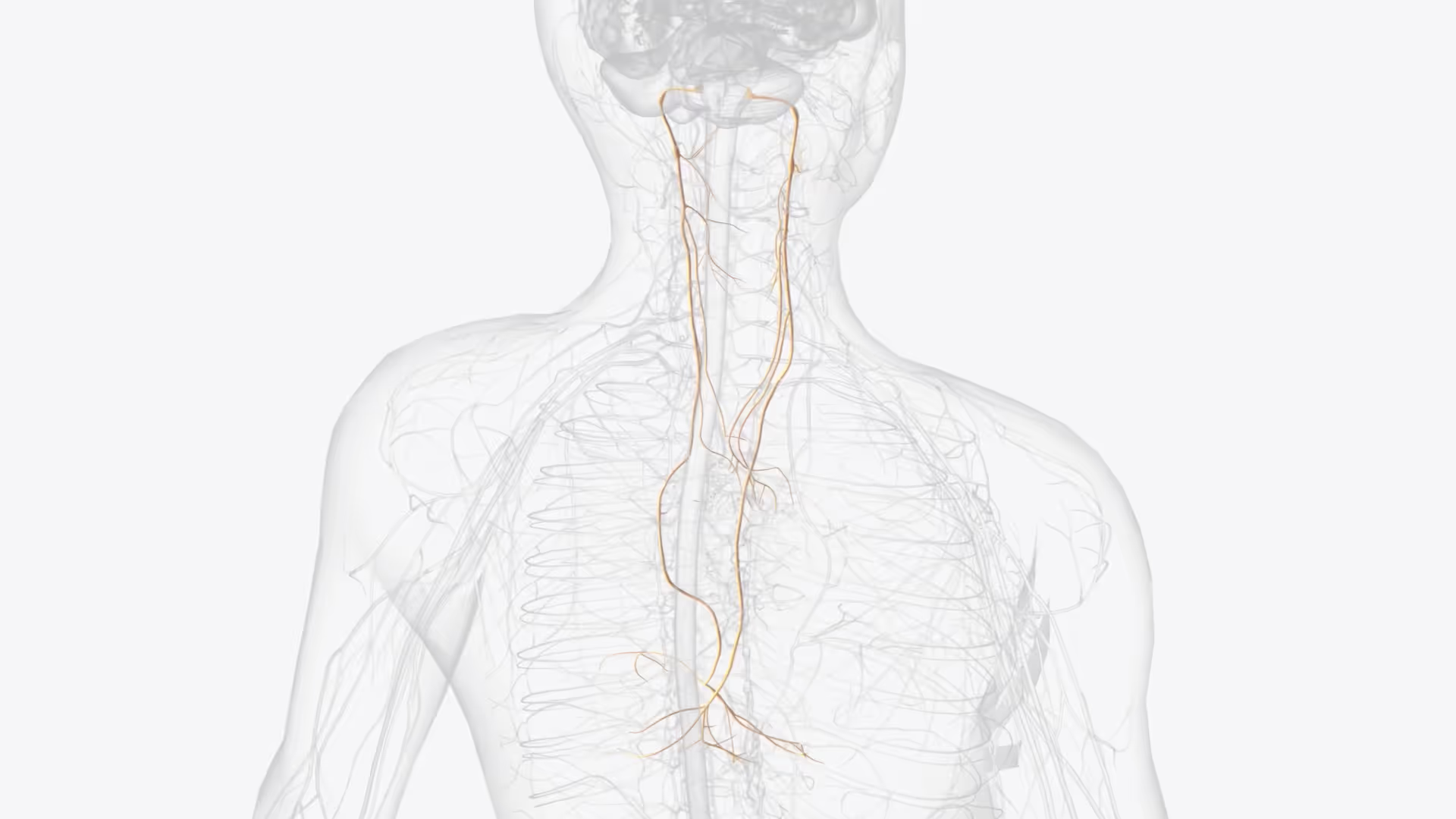
In closed loop neuromodulation, VNS's lack of spatial and temporal selectivity means that the entire nerve is stimulated tonically, causing side effects and suboptimal outcomes. Detecting a seizure biomarker on the vagus nerve could enable closed-loop, on-demand stimulation for epilepsy.
Solution:
We performed a preclinical in vivo investigation to demonstrate the feasibility of detecting a seizure biomarker in both branches of cervical vagus nerves using multi-electrode nerve cuffs. Data was analysed using the velocity-selective recording technique to differentiate fibres conducting at different velocities, and to distinguish afferent from efferent activity.
Result:
We demonstrated increased activation of fibres conducting at 7-15 m/s (Aδ, Aγ) on the right vagus nerve during seizures. While more experiments are required, the findings of the study form the foundation for developing a novel closed-loop neuromodulation system for treating epilepsy.
A step towards more targeted vagus nerve stimulation
Neuromodulation is increasingly used to treat neurological disorders, particularly in patients who do not respond to conventional drugs or are unsuitable for surgery. Among these methods, vagus nerve stimulation (VNS) is commonly applied, typically in a tonic mode that delivers constant stimulation over time. However, this approach often causes side effects and can limit therapeutic efficacy.
To address these limitations, we explored the feasibility of developing a closed-loop VNS system for epilepsy. Such a system would rely on a biomarker of epileptic seizures. We identified such a biomarker on the vagus nerve using a porcine model chosen for its anatomical similarity to humans.
Our approach
TTP's Neurotechnology team developed and carried out in vivo experiments in pigs to demonstrate the feasibility of detecting a biomarker of seizures in both branches of cervical vagus nerves using multi-electrode nerve cuffs. Pigs were chosen for this investigation for the anatomical similarity of their vagus nerves to human ones. The data was analysed using the velocity-selective recording technique, which is also called “spatial averaging”. This approach allowed extracting useful information from noisy data by differentiating between nerve fibres conducting at different velocities, and between afferent and efferent fibres.
Outcome
We demonstrated significantly increased activation of fibres conducting at 7-15 m/s (Aδ, Aγ) on the right vagus nerve during seizures in one animal. This was controlled by comparison with the baseline state, where the activity of fibres conducting at these velocities is largely suppressed. Muscle relaxant and adrenaline injection did not change the VSR spectrum of the vagal activity, confirming that these chemicals did not affect the biomarker.
Impact
TTP’s findings provide the first preliminary evidence supporting the feasibility of detecting a seizure-related biomarker on the vagus nerve. These findings contribute to the basis for the development of a novel medical device capable of delivering closed-loop vagus nerve stimulation based on this primary biomarker. Such a system holds significant promise for reducing stimulation-related side effects, extending the battery life of implanted pulse generators, and enhancing overall treatment outcomes.