
Human-centred design and human factors engineering
At TTP, we design technology around the people who use it – because usability isn’t a feature, it’s fundamental to success. Our Human Factors team fuses behavioural science, engineering and design to create solutions that are safe, effective and embraced in the real world. By applying human-centred design and rigorous human factors engineering across the full development lifecycle, we help clients reduce risk, accelerate progress and deliver commercially viable, market-ready devices.
Early-stage research, ethnography and front-end innovation
We specialise in uncovering unmet needs through contextual enquiry, ethnography and qualitative research, in homes, hospitals and digital environments.
Our front-end programmes reveal deep user insights that inform opportunity discovery, system design and product strategy.
Tools such as patient video diaries, workflow mapping and remote user shadowing help us capture the complexity and variability of real-world behaviours. We apply behavioural science models, including our Design for Adherence model, to predict user behaviour and assess the likely real-world impact of design interventions from the outset.
This enables clients to define more robust user requirements, differentiate their solutions, and reduce the risk of solving the wrong problem.
What could early insight unlock for your project?



Concept development and iteration
Our team works collaboratively across disciplines to translate user insights into practical, manufacturable concepts.
We use the TTP Design for Engagement framework to guide decisions around motivation, habit formation and perceived value, ensuring products aren’t just usable, but genuinely engaging in daily life.
Early iteration with engineers allows us to balance feasibility, cost and user preference, while tools like simulation and cognitive walkthroughs help quickly validate UI, ergonomics and risk controls, providing clear input to make confident, user-informed design choices.


Formative use studies
Formative studies are core to our risk-informed, evidence-based development process.
We plan and conduct user studies across demographics, use environments and device states to identify early usability issues.
Our moderators apply structured observation and cognitive walkthroughs to assess interaction challenges, user satisfaction and training needs.
The results inform design refinements and risk mitigations that improve safety and experience – before validation begins. We offer flexibility in study formats, including remote testing and custom instrumentation, to meet timelines without compromising insight.
User moderation: top 10 mistakes and how to avoid them
Read our ten tips to improve user research in moderation.
Meet us at


Risk analysis and human factors engineering
We embed human factors engineering (HFE) into the risk management process from the outset, aligning with ISO 14971, IEC 62366 and FDA guidance.
Our team conducts use specification, task analysis, use error FMEAs and hazard identification to trace use-related risks across the product lifecycle.
These activities form a core part of our usability engineering file, supporting regulatory submissions, design history files and clinical trial planning. We work closely with design and engineering teams to ensure that risk controls are not only compliant, but also practical, context-aware and grounded in real user behaviour. This integrated approach de-risks development early and builds regulatory robustness, without slowing innovation.
Free Download: Navigating Risk in Medical Device Development
Drawn from decades of experience at TTP, this guide equips you with real-world tools and strategies to keep your development on track while design choices are still flexible. Get your copy.


Summative/validation studies
We design and run summative usability studies to show that products are safe and effective for intended users, uses and environments.
Our evaluations simulate worst-case scenarios to test whether critical tasks can be performed without unacceptable use error.
We generate robust, traceable evidence for regulatory submission, aligned with FDA and MDR expectations.
By assessing training, labelling, design and risk controls, we ensure end-to-end usability. Working closely with your team, we make validation a seamless conclusion to a well-rationalised process – no scramble, just confirmation it works for real users in the real world.


A large proportion of the suffering and economic impact caused by mental health problems is not due to a lack of effective treatments. It is a direct consequence of the lack of access to mental health services due to a demand that far outstrips the availability of trained professionals needed. The scalability of digital therapeutics has the potential to bridge this gap and bring enormous benefits to society.
Dr Jorge Zimbron
Consultant in General Adult Psychiatry, specialising in complex presentations and a diagnosis of personality disorder (PD).


We want releaze™ to be the most user-friendly drainage solution for recurrent pleural effusion, transforming the patient experience in the process. TTP’s proven expertise in medical device development gave us confidence from the outset. Their experience with complex electromechanical systems and taking devices through to market meant they could develop the drainage hub quickly, reliably, and with real efficiency. TTP were flexible in their approach and collaborated early with our manufacturing partner, Phillips-Medisize, to ensure the design was manufacturable from day one. The resulting hub design perfectly complements the simplicity of our catheter system. It is intuitive to use, reassures users and gives patients invaluable independence.
Tim Jones
CEO, SymPhysis
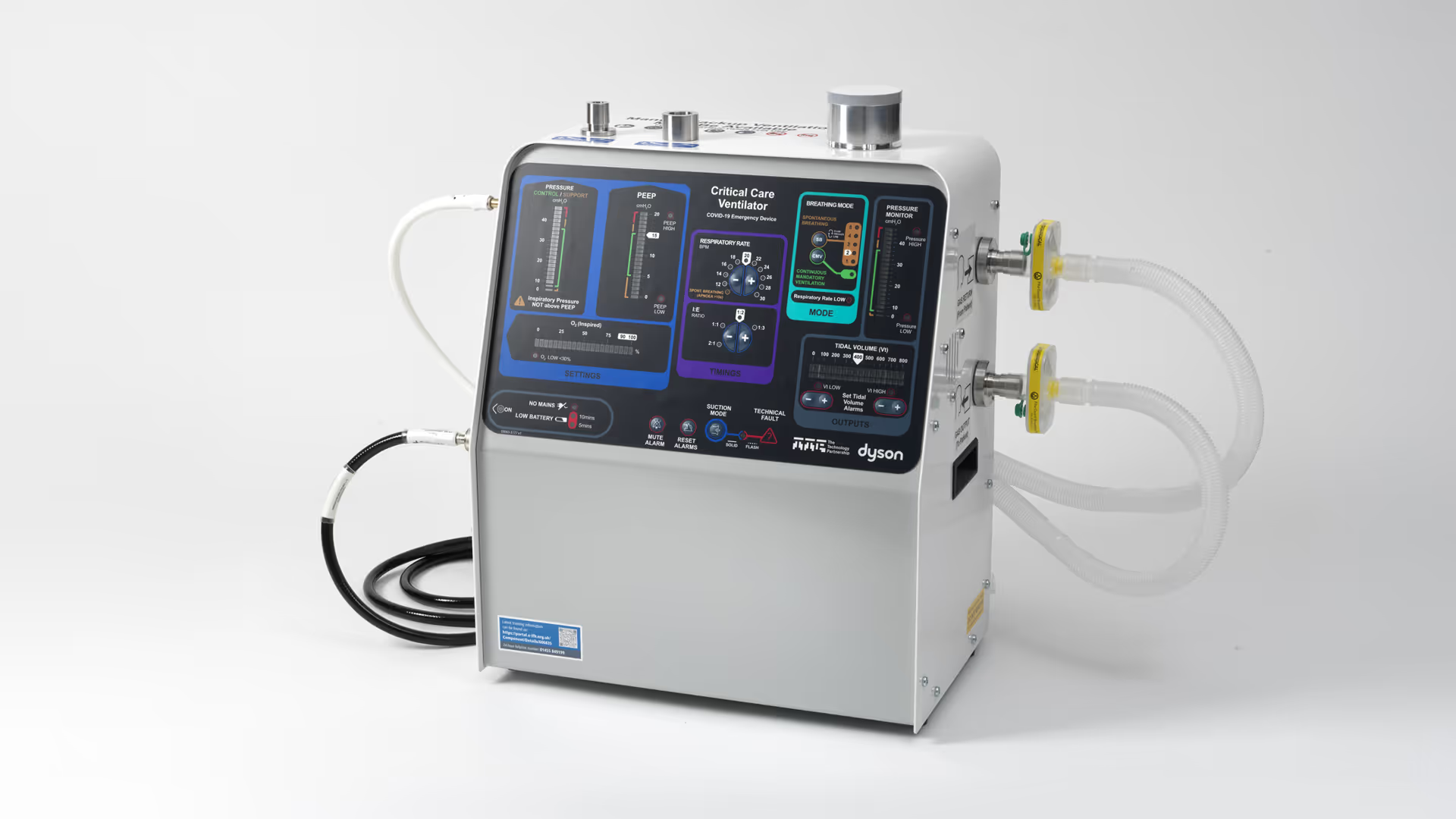

The work to develop the CoVent device to this point has been extraordinary. It is a credit to TTP and your partners for accelerating a process which typically takes years to a number of weeks. I would like to offer my greatest appreciation and thanks for the quite extraordinary endeavour, enterprise and ingenuity shown by all involved in the CoVent ventilator.
Rt Hon Michael Gove MP
Chancellor of the Duchy of Lancaster and Minister for the Cabinet Office
Our approach and capabilities
We deliver across the entire life of a project, from opportunity discovery to production engineering. Discover how our interdisciplinary teams of experts collaborate to tackle the toughest product development challenges.

Our campus and facilities
Our award-winning campus has been designed with a clear vision. To create space which can suport our people and our clients as we develop and deliver the very best technology solution.

Software capability at TTP
Engaged in all stages of software and product development, our software capability at TTP covers the full spectrum—from in-depth analysis and system architecture to prototype design, implementation, and test development.

Manufacturing capability at TTP
Working seamlessly with our development teams, we take clients' products through prototype builds, clinical trials, pilot manufacture and more. Using TTP Manufacturing reduces uncertainty, risk and time to market for our clients.

Meet some of the team

Dan Lock

Steve Gowers

Duncan Young
Talk to us about your next project
We help clients with all stages of their most complex and challenging technology and product development projects.
If you're considering the next steps along your innovation journey, why not get in touch?


















